Kinga Vörös has been interested in the world of medicine since childhood. She moved to Budapest several years ago to study pharmaceutical engineering and is currently conducting research in the HCEMM-SU Neurobiology and Neurodegenerative Diseases Research Group at Semmelweis University’s Institute of Translational Medicine. Building on a strong foundation in chemistry and biology in her hometown, she gained knowledge as a bioengineer of the chemical processes of living organisms, drug development, drug effects, and testing. “I can still use what I learned back then in my daily work,” the Semmelweis PhD student and researcher emphasized.
Kinga Vörös has been interested in the world of medicine since childhood. She moved to Budapest several years ago to study pharmaceutical engineering and is currently conducting research in the HCEMM-SU Neurobiology and Neurodegenerative Diseases Research Group at Semmelweis University’s Institute of Translational Medicine. Building on a strong foundation in chemistry and biology in her hometown, she gained knowledge as a bioengineer of the chemical processes of living organisms, drug development, drug effects, and testing. “I can still use what I learned back then in my daily work,” the Semmelweis PhD student and researcher emphasized.
Her passion for cell biology began before university, when she won a six-week scholarship to the University of Arizona in the United States, where she was able to visit numerous research laboratories. All of this had a major impact on the fact that cancer research became the focus of her interest as she returned home. In her university thesis, she investigated the cellular anti-cancer effects of a blood substitute. After gaining industrial and institutional experience in the world of research, she applied for PhD training at Semmelweis University, where she became more closely acquainted with the world of neurons. As she said, explaining the background to her decision:
Cancer research is a beautiful field, but neural networks, the functioning of neurons – how we think through them, how impulses travel through nerve cells and axons, and research into neurological disorders – that is a very different world. We know of many types of neurodegenerative diseases, and research related to aging has also become very important to me. I felt that this was what I needed to focus on now.
Their research is investigating the therapeutic potential in Huntington’s disease of a calcium channel blocker originally developed for the treatment of hypertension, using a special preclinical model. The drug has been on the market since the 1980s; there is data in the literature on its experimental application in animal models, and it has been used in clinical medicine long enough for its effects to be well known, so it may be suitable for reuse in Huntington’s disease patients.
Their scientific investigation focuses on a comparison of a clinical and a preclinical experiment in collaboration with the University of Cambridge, she outlined. A cohort of 18 Huntington’s disease patients and seven healthy controls was selected for the repurposing testing of the medication felodipine. The Semmelweis laboratory reprogrammed fibroblast (skin sample) cells from the entire cohort into directly induced neurons (iN) so that the resulting nerve cells retain the genetic and age-related characteristics of the donor. Patients in Cambridge received the antihypertensive drug for one year, and its tolerability and safety were evaluated. At the same time, at Semmelweis University the effect of the same drug was tested on induced neurons created from fibroblasts: how it works at the cellular level, how different functions change, with particular regard to the mechanism of autophagy (intracellular degradation process).
A characteristic feature of Huntington’s disease is that, once it sets in, an accelerated aging process begins in the neuron cells of previously healthy patients, leading to the death of certain neurons, which causes cognitive, motor, sensory, and balance disorders. The process is irreversible, and there is currently no known cure; only the symptoms can be treated. As in other neurodegenerative diseases such as Alzheimer’s or Parkinson’s disease, rapid aging can be observed in the nerve cells. In addition, autophagy, an important, evolutionarily conserved lysosomal degradation process, does not function properly in neurons, causing harmful proteins to accumulate and aggregate in certain areas of the brain.
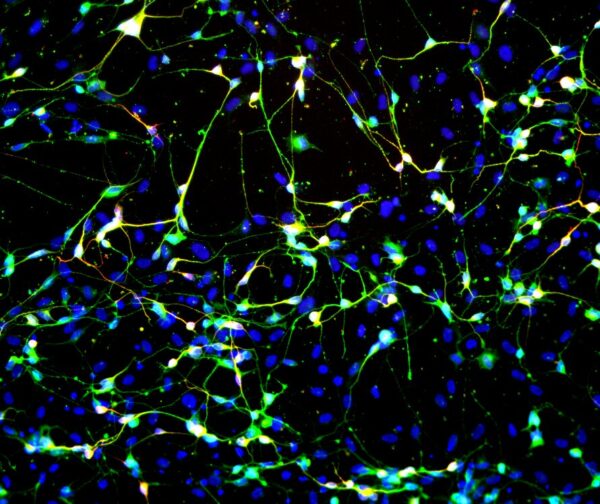
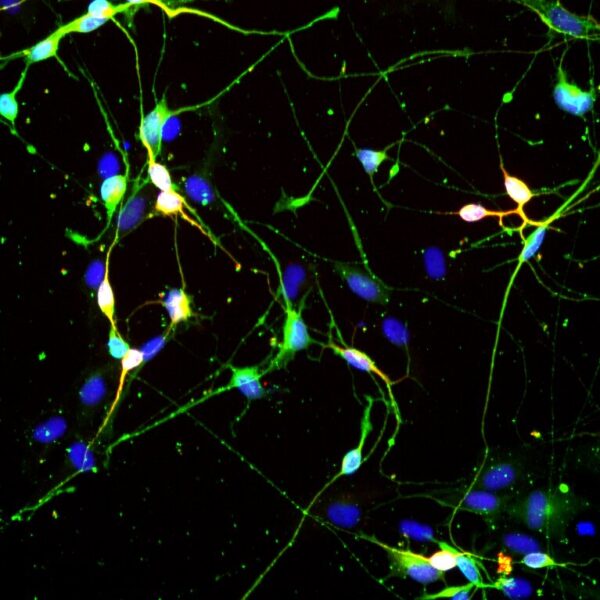
Induced neurons reprogrammed from human skin cells in just four weeks have a similar structure to brain neurons, consisting of a cell body and axons. They become visible under a microscope and can be distinguished from other cells after neuron-specific staining (they fluoresce green). A special device can be used to measure the various morphological characteristics of the nerve cells, which are then compared with the results from healthy control subjects.
The researcher said that the planned experimental phase was now largely complete, and they were currently working on the patent process and publishing the data. “We will certainly hear more about this after publication,” she added. The goal is to use their model to aid clinical research and drug testing, thus improving their effectiveness, which is extremely low in the case of Huntington’s disease: The success rate of phase 3 drug trials is around 3 percent. “In the future, we plan to expand our research to include several other neurodegenerative diseases, such as Alzheimer’s and Parkinson’s,” she pointed out.
“It feels good to receive recognition and professional feedback,” she said, noting that she had recently been invited to a prestigious conference in Germany. She added that cell biology research is not an easy profession; despite the many joys, there are also difficulties when cells do not do what the researcher wants them to do, or when a process lasting weeks has to be restarted because of some minor detail. “Working with neurons is the most difficult as they are sensitive, non-dividing cells that must be handled with great care. I like to say that they are like children: We change and feed them, and perhaps it even matters what we think and do when we are around them. We can’t just go home without taking care of them; in fact, they are always the first to eat, and only then do we,” she said, providing insight into the world of cell laboratories.
My work as a researcher here at Semmelweis is what I have always wanted to do. The topic is very relevant, and the results and outcomes are tangible. I have worked in several different areas of research, but I feel that here I can see first-hand how our work is being put to use in helping people.
Róbert Tasnádi
Translation: Dr. Balázs Csizmadia
Photos by Boglárka Zellei – Semmelweis University