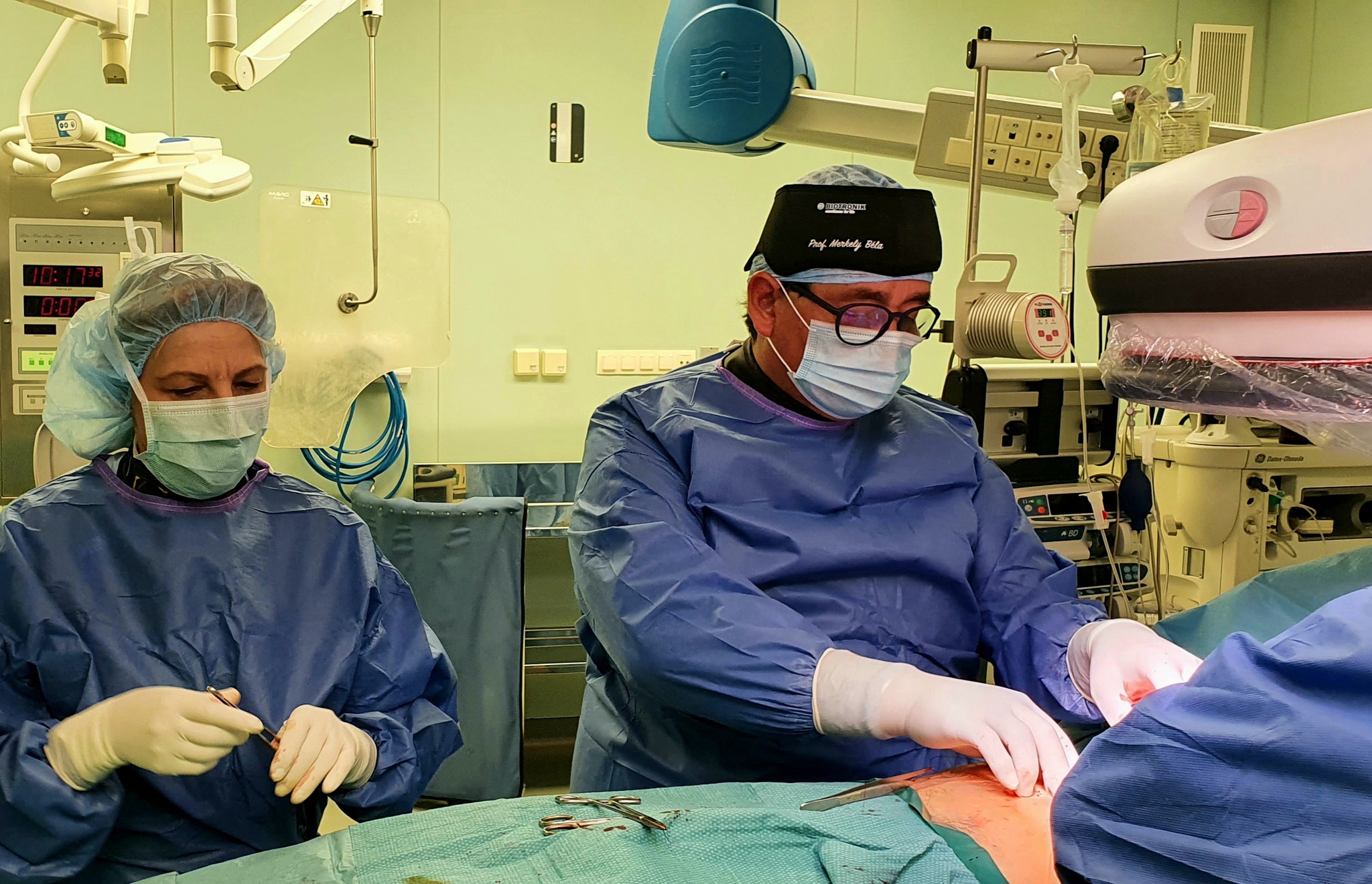
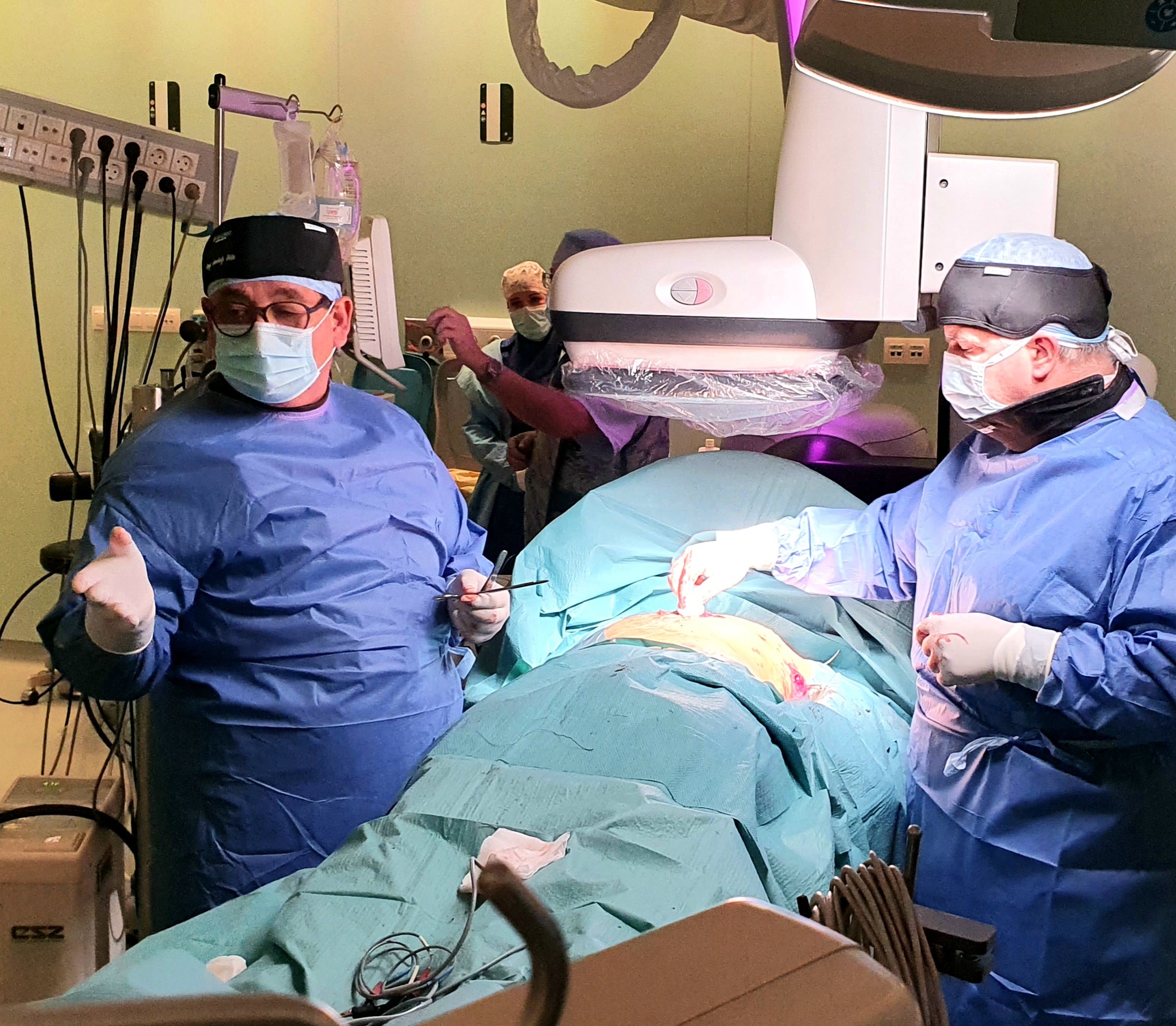
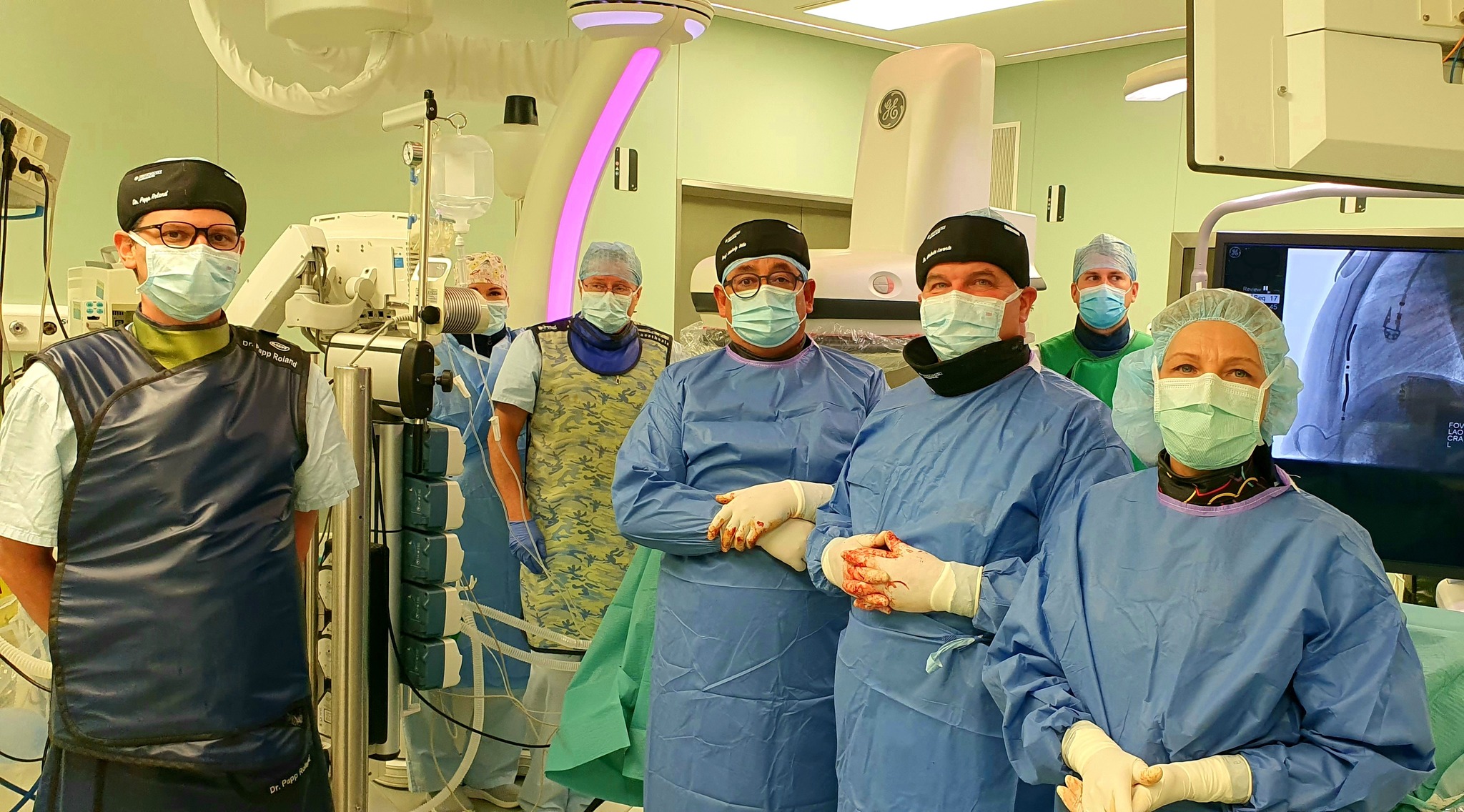
In addition to using less energy, the new type of so-called substernal extravascular ICD (Implantable Cardioverter Defibrillator) device, which went on sale worldwide in September 2023, eliminates dangerous heart rhythm disorders more effectively, in the development and clinical trials of which the Semmelweis University’s Városmajor Heart and Vascular Center specialists played an active role. The Center was also involved in the development and the subsequent clinical tests since the team lead by Dr. Béla Merkely and his colleagues, Dr. Levente Molnár and Dr. Roland Papp had sufficient practical knowledge to be the first to perform a successful extravascular ICD implantation (EV ICD) in October, not only in Hungary, but also in the CEMA region, which includes Central and Eastern Europe, the Middle East and Africa.
Since the operation, the 19-year-old patient diagnosed with thickening of the heart muscle has been doing well, had no heart rhythm problems and has not been hospitalized, said Chief Clinical Physician Dr. Levente Molnár. In the middle of December, the second successful operation was also performed when another younger patient at risk of sudden cardiac death received a new type of implantable defibrillator under anesthesia.
Doctors typically use this device in two cases: if, based on the patient history, the risk of sudden cardiac death is very high over a five-year period, and the EV ICD is implanted for as a preventative measure; or with the aim of secondary prevention if the patient has already overcome a life-threatening rhythm disorder. In the case of life-threatening heart rhythm disturbances, the risk of sudden cardiac death is not primarily due to age, but rather the pathological background, for example, the patient had a heart attack in the past, or weak left ventricular function is diagnosed, or sudden cardiac arrest has already occurred in the family.
During the collaboration with one of the world’s leading medical device manufacturers and the approximately 5-6 years of research leading to the development of the instrument, the Center participated in two major studies. In the acute phase study, the positioning of the electrode under the sternum was tested in an international study – they were world pioneers in this regard – and in the other study, the device was tested when already implanted.
The pre-implantation CT analysis, which can be used to map the anatomical parameters under which the insertion and operation of the device is optimal and safe, was recommended by the specialists of the clinic, and in the end, it brought valuable data not only for the developer company, but also for the other partners participating in the study
— said Dr. Béla Merkely, rector of Semmelweis University, director of the Városmajor Heart and Vascular Center. Thanks to this, it became clear that there are anatomical parameters in which heart rhythm regulation is effective only with maximum energy use. The results were summarized in an article published in the journal Europace by the specialists participating in the tests. (Europace. 2022 May 3;24(5):762-773. doi: 10.1093/europace/euab243., The extravascular implantable cardioverter-defibrillator: characterization of anatomical parameters impacting substernal implantation and defibrillation efficacy. Levente Molnár, Ian Crozier, Haris Haqqani, David O’Donnell, Emily Kotschet, Jeffrey Alison, Amy E. Thompson, Varun A Bhatia, Roland Papp, Endre Zima, Ádám Jermendy, Astrid Apor, Béla Merkely).
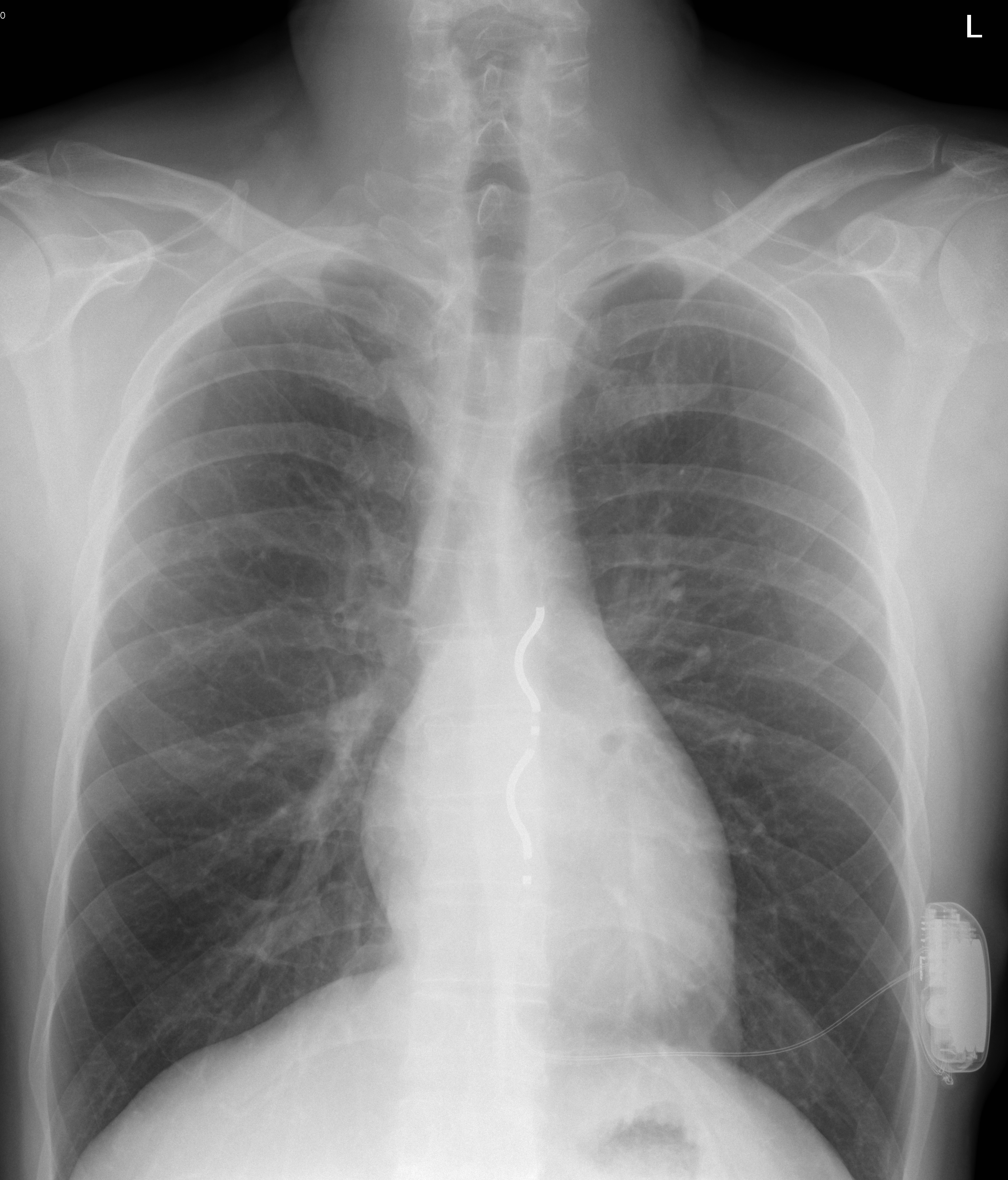
The novelty of the EV ICD device lies in the fact that the electrode that controls the functioning of the heart is implanted behind the sternum, while the device itself is located under the left armpit. (The lead of a conventional ICD is inserted through a vein into the heart cavity and attached to the wall of the right ventricle, and the device is implanted under the left collarbone. Another alternative device, the subcutaneous ICD, places the pacemaker electrode under the skin outside the rib cage, and the rest of the device is located in the left armpit.) The electrode of the new type of EV ICD is thus closer to the heart, so the energy used to control the operation is smaller, but so is the battery that operates the device. In addition, the EV ICD – like conventional ICDs – is able to direct the abnormal function towards a normal rhythm in the event of a heart rhythm disturbance through the so-called anti-tachycardia pacing function with a series of small electrical pulses, not only with shocks, said Dr. Levente Molnár.
Among other things, the lower energy consumption is beneficial for patients because the battery life of the device is almost the same as that of a conventional ICD, but longer than that of a subcutaneous ICD, up to 11.5 years. In addition, unlike conventional defibrillator electrodes that can be implanted in the right ventricle, the EV ICD electrode is fixed, so there is less chance of the electrode breaking. It is also beneficial for the patient that since the electrode does not penetrate the heart, the risk of infection is lower both during implantation and in the case of interventions that may become necessary later.
Melinda Katalin Kiss
Translation: Mária Sánta
Photo: Semmelweis University