 “Pathologists traditionally examine the tissues and blood vessels that make up human organs using histological procedures, preparing sections of micron (μ=1/1000 mm) size, staining them, and examining them on a microscope slide,” the researcher says, explaining the background to her work. “Due to its level of detail, this is a process with good diagnostic value, but it is time-consuming and requires a sample taken from the tissue in question,” she adds. “With imaging equipment, such as the state-of-the-art photon-counting CT scanner used at OKK, we can visualize organs non-invasively using X-rays, revealing a microscopic anatomical image of the area examined as well as any organ lesions. With ever-higher image resolution, tumorous changes and deposits (plaques) on the coronary arteries of the heart can be detected at an early stage,” the researcher explains.
“Pathologists traditionally examine the tissues and blood vessels that make up human organs using histological procedures, preparing sections of micron (μ=1/1000 mm) size, staining them, and examining them on a microscope slide,” the researcher says, explaining the background to her work. “Due to its level of detail, this is a process with good diagnostic value, but it is time-consuming and requires a sample taken from the tissue in question,” she adds. “With imaging equipment, such as the state-of-the-art photon-counting CT scanner used at OKK, we can visualize organs non-invasively using X-rays, revealing a microscopic anatomical image of the area examined as well as any organ lesions. With ever-higher image resolution, tumorous changes and deposits (plaques) on the coronary arteries of the heart can be detected at an early stage,” the researcher explains.
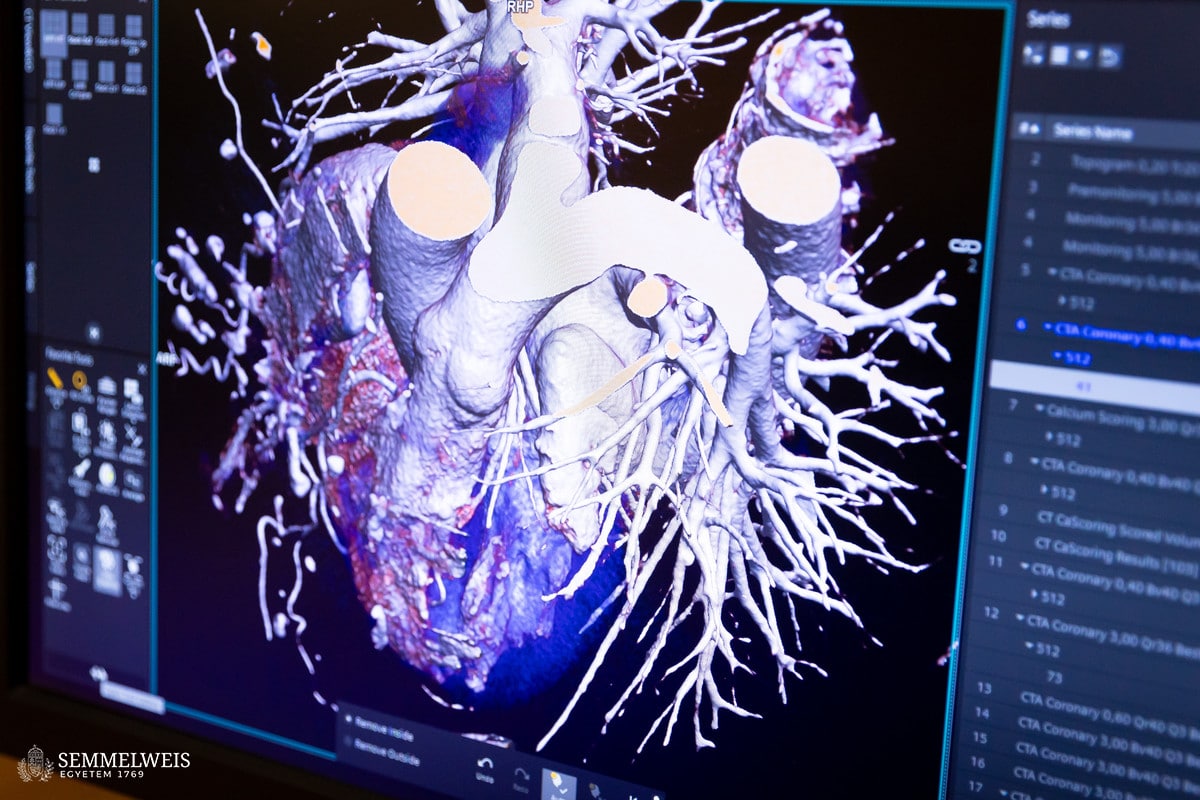
“Each tissue absorbs X-rays differently: Bones appear bright, while fat appears dark and black on a grayscale CT image. The different types of plaque that cause coronary artery disease can be distinguished by their shades: Calcified plaques appear bright, non-calcified, fatty plaques appear as dark spots, while so-called ‘mixed’ plaques contain both components. However, histologically, there are six types of plaque and other markers of high risk, but these cannot be distinguished on CT images due to resolution limitations,” she points out, explaining the differences.
The basic idea for the research came from her supervisor, Dr. Pál Maurovich Horvat, Director of the Medical Imaging Center, who had previously conducted postgraduate research at Harvard University in Boston. Encouraged by him, Dr. Lili Száraz began to review these studies and then reproduce them using the latest imaging techniques. The essence of this is that the conditions suitable for clinical CT examinations are created on post-mortem (deceased) organs provided by the Department of Pathology and Experimental Cancer Research, CT images are taken with different dosage-related machine settings, and then images of histological sections are superimposed onto the CT slices, comparing them to examine where the six known histological plaque types are visible on the CT images (see figures).
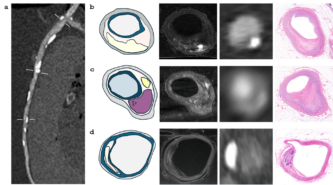
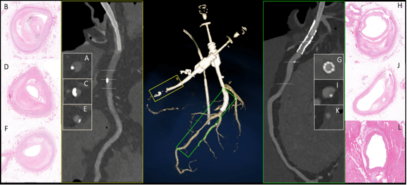
“The advantage of this examination method is that you can try anything on the post-mortem heart that is not possible in the clinic, including various contrast agents that have not yet been introduced or protocols that are questionable from a radiation protection point of view,” she explains. The comparison can reveal the lowest dose setting that still provides an adequate diagnostic image. It can examine vasoconstriction and plaques to see if everything is the same as in the histological section or if the CT image shows something different. This is because the imaging algorithm of the equipment may overestimate the degree of stenosis, which is not confirmed by the catheter or histological examination. This also allows for the testing of routine protocols.
“Under our center’s agreement with the Department of Pathology and Experimental Cancer Research, as soon as an organ is available, we transfer it to the center. In the experimental operating room of the surgery, under the supervision of radiologist Dr. Ibolyka Dudás, my job is to prepare and fix the organ and then perform the CT scan. In such cases, imaging is performed on four scanners: a conventional, so-called energy-integrating detector, a single and dual X-ray source, and a photon-counting CT device. This covers all the major manufacturers’ scanners,” she said, giving an insight into the practical work. She added: “The time factor is very important here, we have to act quickly before the various post-mortem changes begin in the organ, so once the work starts, we work on it all day and night until we have finished.”
“What we are already using these tests for is the further development of micro-CT machines (microscopic CT: works on the same principle as traditional CT but provides much higher resolution images). With its high resolution, micro-CT can produce histological-quality images, which could replace traditional, time-consuming pathological section imaging.”
The researcher would like to see a large database compiled from the image series, on which a histology-based plaque composition analysis software could be built. “This requires a large number of elements, which means that a lot of samples need to be examined. The next step is to prove that it works reliably in practical clinical conditions when examining patients. To do this, the program will need to be validated in a large, multi-phase clinical trial,” she emphasizes.
“This is a unique project; I don’t think it could be done anywhere else in the same way as we are doing it here. Post-mortem heart research is rare on a global scale anyway, as I have learned at conferences, where our presentations are met with keen interest. The advantage of our infrastructure is that, within a university, the institutes working together are located close to each other, which is a significant advantage for us in terms of the rapid delivery of test samples. In addition, at our center we have access to several types of state-of-the-art imaging equipment. These three factors represent an exceptional combination, offering great opportunities for research to progress in this way,” she pointed out.
Dr. Lili Száraz emphasized that, despite all its challenges, the research was a great motivation for her:
I believe that this work contributes to even more accurate and faster diagnoses. For example, the analysis software could provide a much more accurate assessment of the risk of vasoconstriction, and we would also be able to better monitor the effects of medications. With our software, we would also be able to see what changes are taking place within a plaque, and in another project, we are looking for answers to how short-term cholesterol-lowering treatment affects plaques.
For further articles introducing last year’s Semmelweis Innovation Award winners, click on the links below:
Research aimed at developing vitamin C eye drops wins Semmelweis Innovation Award
Kinga Vörös: I can see first-hand how our work is being put to use at the bedside
Róbert Tasnádi
Translation: Dr. Balázs Csizmadia
Photos by Boglárka Zellei – Semmelweis University