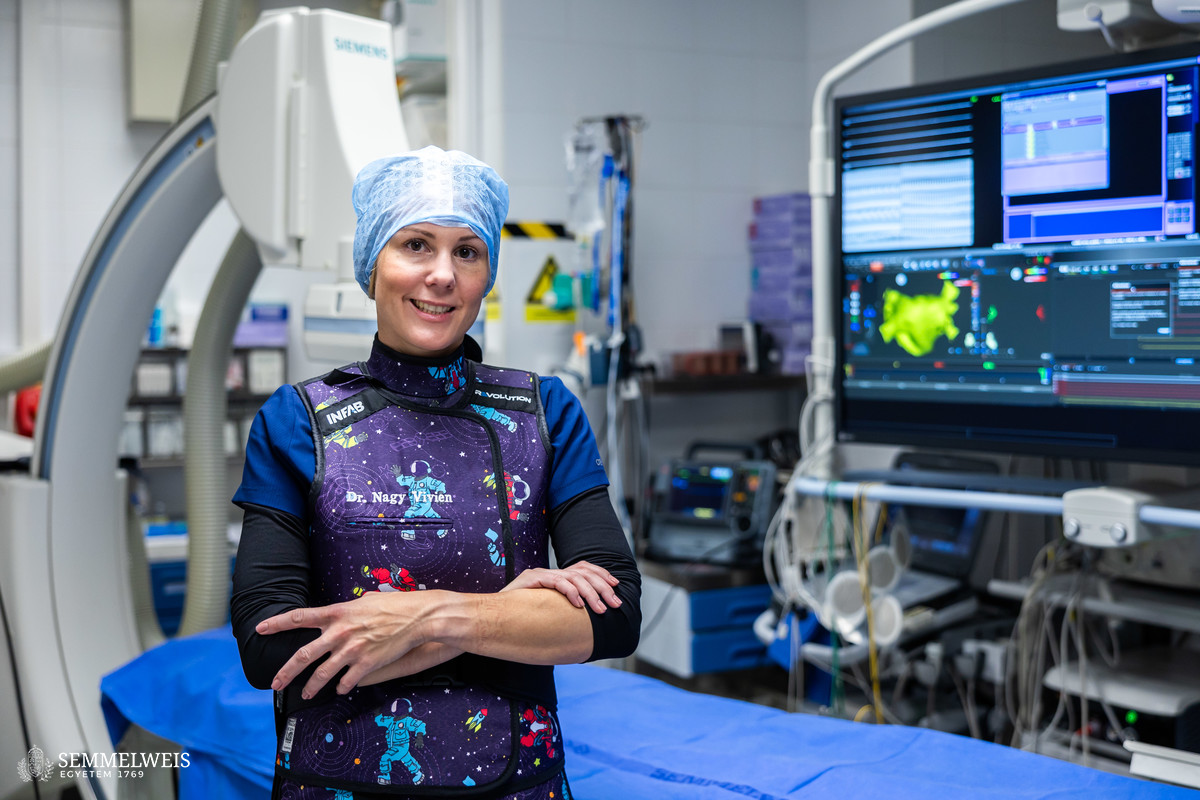
Semmelweis University is also an active participant in the Hungarian to Orbit (HUNOR) Astronaut Program, providing medical expertise, including the medical selection and training of astronauts, as well as monitoring their health status. The physical condition of Hungarian astronaut Tibor Kapu is assessed by Dr. Klaudia Vivien Nagy, Associate Professor at the Városmajor Heart and Vascular Center, who is a space surgeon certified by the European Space Agency (ESA). Besides her involvement, Semmelweis University is also affiliated with two research projects.
The Telemetry System of Space Health (TESH) project aims to investigate health problems during space travel. This is a multimodal, non-invasive telemedicine trial focusing on early cardiovascular and neurovascular changes during spaceflight, said Dr. Klaudia Vivien Nagy.
Even during short-term missions, symptoms such as blood pressure fluctuations or imbalance can occur as a result of the adaptation process of the cardiovascular and vestibular organs, which help maintain balance. The experiment aims to better understand these processes and, in the long term, to use the findings to develop a telemedicine system that can help to continuously assess and even automatically monitor the astronauts’ health.
During the test, subjects wear a special smart shirt that continuously logs heart rate (ECG), breathing, body temperature, movement, and heart rate variability. In addition, a 24-hour blood pressure monitor, ultrasound scans of the heart and blood vessels, video balance tests, and fundus imaging are also part of the scientific research, added Dr. Klaudia Vivien Nagy. The phenomena studied by the project do not only occur during spaceflight, similar changes can also happen during ageing. Thus, a telemedicine tool developed in space research could also be suitable for use on the ground, allowing remote monitoring of patients, early detection of medical conditions, and alerting the medical staff.
Dr. Klaudia Vivien Nagy stressed that the results would not only promote the health of astronauts but could also contribute to the advancement of clinical medicine and diagnostics.
One of the main developers of the project is Semmelweis University, with the lead institution European Knowledge Center and the Budapest University of Technology and Economics (BME), the German Aerospace Center (DLR), and the Canadian Space Agency (CSA) as collaborating partners.
Semmelweis University contributed to the selection and training process of the Hungarian to Orbit (HUNOR) Astronaut Program as a medical support institution. Dr. Béla Merkely, Rector of Semmelweis University and Head of the Department of Aviation and Space Medicine, is in charge of the medical expert tasks within the HUNOR Program’s Steering Board. Dr. Klaudia Vivien Nagy, Assistant Professor at Semmelweis University’s Heart and Vascular Center, was the first in Hungary to complete the European Space Agency’s (ESA) accredited space medicine training, thus becoming the first person from Hungary whom the ESA certified to practice space medicine in support of European astronauts.
The other experiment is END-SANS, which tests the feasibility of a new drug delivery method using artificial nanofibers. A successful active agent-free test could lead to the development of targeted ophthalmic therapies in space and on the ground.
“When the university was doing the health assessment part of the selection process, the specialists at the Department of Ophthalmology were assigned to perform the basic eye examination. That was when I started to study the actual strain on the astronauts’ eyes, and that’s when we started to brainstorm with Dr. György Tibor Balogh on ways to simplify the treatment of potential ophthalmological problems during space flights,” Dr. Zoltán Zsolt Nagy, Head of the department, recalled the beginnings. In fact, blood flow and lymphatic circulation are altered in microgravity, which can cause edema in the eyes and the brain. Altered circulation leads to a variety of ophthalmological conditions, one of the most common being altered refraction within the eyeball, essentially hypermetropia, which makes it difficult to handle instruments and may cause edema in the fundus. The latter is routinely treated with an eye drop containing the active substance Nepafenac, but in microgravity, it is not possible to administer the active substance into the eye using the traditional way, i.e., by dropping it into the eye. It is a lucky coincidence that Professor Balogh’s spouse, Dr. Diána Balogh, is an assistant professor at BME, conducting research on materials science, including the attachment of active substances and proteins to solid carriers, which is where the idea to consider nanofiber technology came from.
The research aimed to create a polymer-based unit of nano-sized fibers that dissolves on the surface of the eye, is non-toxic, and can trap the appropriate drug substance and deliver it to the desired place of action.
“Not only were we able to create such an inserter, the size of a grain of rice, but we also managed to get the approval of Axiom and NASA to let astronauts test the samples without an active ingredient,” said Dr. György Tibor Balogh, Head of the Department of Pharmaceutical Chemistry. This device is inserted by pulling down the lower eyelid, like putting on a contact lens. It causes a mild and brief foreign body sensation – but not tearing, unlike traditional eye drops, as the excipient added to the polymeric fiber ensures adhesion to the eye surface and accelerates integration with the tear film – and then dissolves on the surface of the eye and is absorbed locally. The material created by electrostatic fiber formation can be post-formed and precisely dosed under aseptic conditions. The active pharmaceutical ingredient, which is only a few millimeters in size, can then reach the target area much more efficiently. In addition, the rapid solubility and absorption of the nanofibrous carrier, due to its huge specific surface area, means that only a third or a quarter of the active ingredient is needed compared to an eye drop.
In cooperation with Spinsplit Ltd., the Department of Pharmaceutical Chemistry, and the Budapest University of Technology and Economics, the sterile samples were manufactured and shipped to the United States. Testing on the ground is soon to follow, with clinical trials of inserts in healthy volunteers starting at the Department of Ophthalmology. This will be the first clinical trial for which a sterile sample will be produced at the Faculty of Pharmaceutical Sciences, emphasized Dr. György Tibor Balogh.
The professors believe that in the longer term, many routine ophthalmic interventions and treatments could use nanofiber inserts as a painless means of drug delivery, such as administering painkillers, dilators, or anti-inflammatory eye drops.
“We are hopeful that the successful experiment will not only contribute to the long-term health of astronauts, drawing international attention to the results of nanoscience in addition to Hungarian ophthalmology and pharmaceutical chemistry, but will also make the treatment of patients on earth easier and more cost-efficient,” noted Dr. Zoltán Zsolt Nagy.
Eszter Keresztes, Melinda Katalin Kiss
Translation: Judit Szabados-Dőtsch
Photos by Bálint Barta, Boglárka Zellei – Semmelweis University