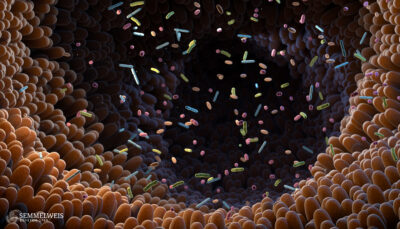
Researchers at Semmelweis University are investigating why and how the most frequently used painkillers, known as NSAIDs, can cause dysbiosis – an imbalance in the gut microbiota. The intestines hold over a trillion microorganisms, including bacteria, viruses, and fungi. These are called the gut microbiota and are essential for the body’s healthy functioning.
 “We have long known that excessive use of non-steroidal anti-inflammatory drugs can disrupt the balance of the gut microbiota. We are now looking for the underlying mechanisms, which are only partially understood,” says Dr. Zoltán Zádori, leader of the Gastrointestinal Research Group at the Department of Pharmacology and Pharmacotherapy at Semmelweis University.
“We have long known that excessive use of non-steroidal anti-inflammatory drugs can disrupt the balance of the gut microbiota. We are now looking for the underlying mechanisms, which are only partially understood,” says Dr. Zoltán Zádori, leader of the Gastrointestinal Research Group at the Department of Pharmacology and Pharmacotherapy at Semmelweis University.
NSAIDs are among the world’s most used medications, with over 30 million people taking them daily, the researchers wrote in a review study published in the journal Pharmacology & Therapeutics.
Some diseases, such as chronic joint inflammation affecting the spine and limbs or rheumatoid arthritis, are associated with an imbalance in the gut microbiota and an overgrowth of certain bacteria, which are linked to the development and worsening of these diseases, says Dr. Zádori.
“We found that these bacterial deviations are similar to those caused by NSAIDs.
This raises the possibility that drug-induced gut dysbiosis could worsen the underlying diseases and limit the therapeutic effect of non-steroidal anti-inflammatory drugs in the long run,
adds Dr. Zádori. However, since these processes all interact, it’s challenging to identify the original cause.
“We currently assume several mechanisms behind the NSAID-induced dysbiosis, including the inflammation of the intestinal mucosa, changes in the pH or motor activity of the intestines, or changes in the bile acid composition. It’s also possible that the NSAIDs’ antibacterial properties directly disrupt the balance of the gut microbiota. These processes are closely linked, so it’s difficult to determine which caused which. For example, reduced bile function can lead to an overgrowth of intestinal bacteria. At the same time, dysbiosis can also change the quantity of certain bile acids,” explains the lead researcher.
NSAIDs usually affect the small intestine, causing hardly noticeable mild inflammation with symptoms like abdominal pain, diarrhoea, or anaemia. In rare, severe cases, it can also lead to bowel perforation.
The gut microbiota plays a fundamental role in body regulation, including balancing the sugar and energy levels, aiding the immune system, and regulating the sensitivity and movement of the intestinal wall. Therefore, an imbalance in the gut microbiota can contribute to the development of various diseases, including irritable bowel syndrome, hormonal disorders, cardiovascular, autoimmune, and psychiatric diseases. Generally, a healthy lifestyle can improve gut health by choosing a proper diet and exercising.
Dr. Zádori states that no rule fits all regarding the safe threshold of NSAID intake and gut health. There are significant individual differences in sensitivity to NSAIDs, partly because the gut microbiota composition highly depends on several other factors, including the patient’s age, lifestyle and health status.
The Hungarian researchers are also seeking ways to counteract or mitigate the harmful effects of NSAIDs. They are experimenting, among others, with cannabinoids, which have already been effective in treating some intestinal issues, such as irritable bowel syndrome.
Photo: Balint Barta – Semmelweis University; Cover photo (illustration): iStock – Roman Valiev