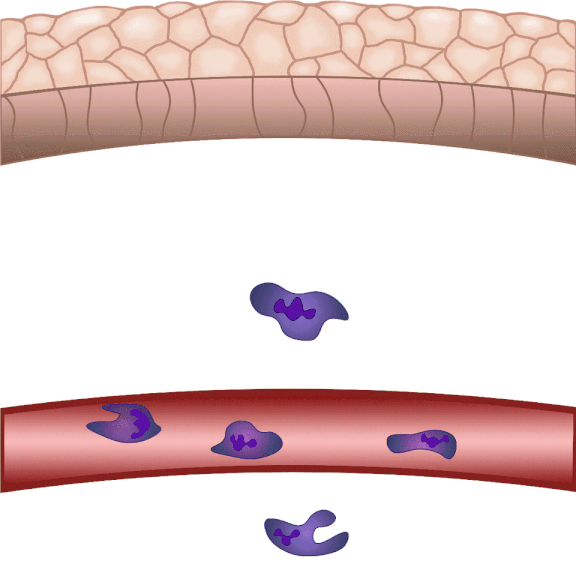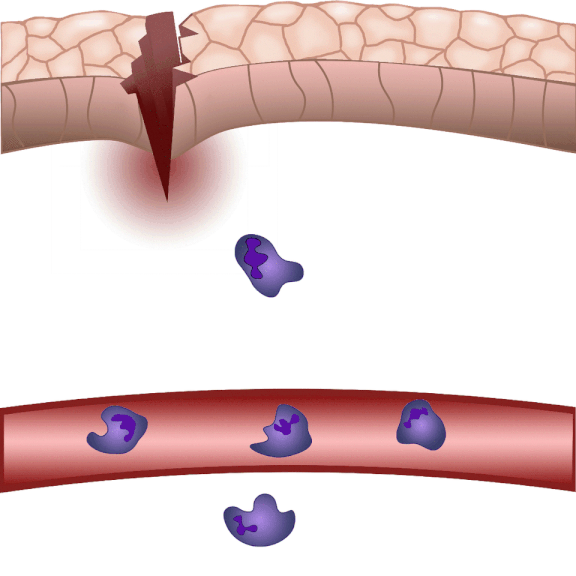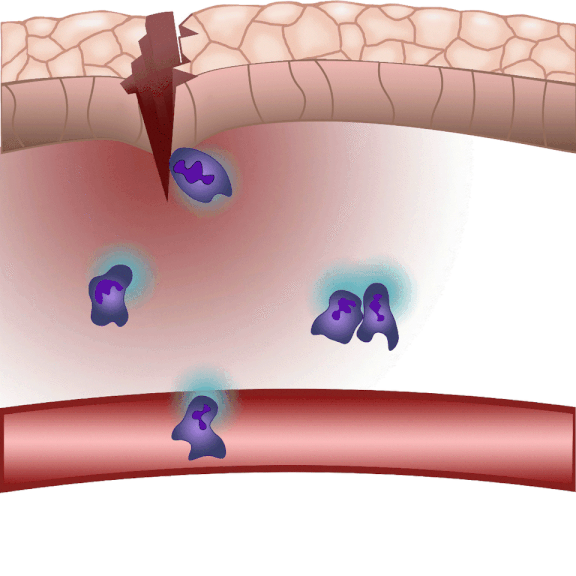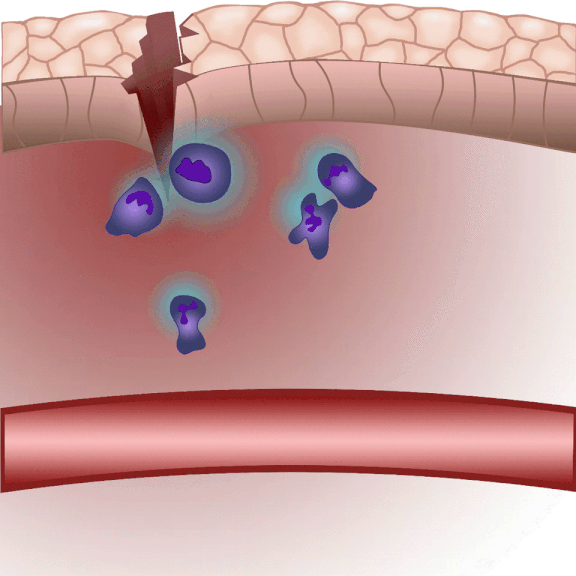
In the event of tissue damage, the body mobilizes its defence mechanisms against potential pathogens from the outside world within minutes. Nobel laureate Ilya Ilyich Mechnikov, while examining starfish larvae under the microscope, noticed that cells migrate in direction of the wound after injury – and discovered immune cells, now known as phagocytes. “Although the basic phenomenon has been known for nearly 150 years, many details of this finely regulated process are still not well understood. In our experiments on zebrafish, we wound the caudal fin of 2-3 millimeter long zebrafish larvae, and use microscopy to visualize the regulatory processes that allow white blood cells to migrate to the wound site within minutes to protect the body from pathogens and promote wound contraction and subsequent healing,” explained Dr. Balázs Enyedi.
For centuries, researchers have used different model systems to understand biological processes. Zebrafish were introduced to the world of research in the 1960s by a Hungarian-born American molecular biologist, George Streisinger. Initially, zebrafish were used mainly for developmental biology studies, as they reproduce by external fertilization, so it is easy to observe the development of the organism from a single-cell stage, even under a light microscope. Researchers began to use them modelling human diseases in the 2000s, initially mainly for monogenic genetic diseases, then more widely, including inflammation and tumour biology. Dr. Balázs Enyedi’s team chose zebrafish for their research because they have similar cellular inflammatory processes to those in mammalian and human systems. They take advantage of the fact that their larvae are transparent, making them particularly easy to examine under a microscope. In addition to that, zebrafish can be genetically modified, so that in the lab they can incorporate transgenes, called fluorescent sensors, into the fish’s genetic pool, allowing them to track the molecules they want to study in larvae under the microscope.
 The cell movement triggered by environmental chemical stimuli is called chemotaxis, and the molecules that enhance cell migration in the direction of the chemical stimulus are called chemoattractants. Following injury, chemoattractants are produced or released in the injured tissue that induce white blood cell migration and local inflammation. So far, these molecules could not be observed in real time, we did not know exactly when and from which cells they were released, and how far they traveled from the source,” said the associate professor. The new method visualizes and measures one of the molecules that regulates inflammation, leukotriene B4, in real time, making it possible to study the location and tissue distribution of a chemoattractant release in inflammation for the first time.
The cell movement triggered by environmental chemical stimuli is called chemotaxis, and the molecules that enhance cell migration in the direction of the chemical stimulus are called chemoattractants. Following injury, chemoattractants are produced or released in the injured tissue that induce white blood cell migration and local inflammation. So far, these molecules could not be observed in real time, we did not know exactly when and from which cells they were released, and how far they traveled from the source,” said the associate professor. The new method visualizes and measures one of the molecules that regulates inflammation, leukotriene B4, in real time, making it possible to study the location and tissue distribution of a chemoattractant release in inflammation for the first time.
In effect, we have developed a new pair of glasses to visualize a key inflammation-regulating molecule,
pointed out Dr. Balázs Enyedi.
“When injured, tissues produce an ‘inflammatory cocktail’ – a reaction that triggers a flood of white blood cells to the affected area. The greater the injury or the more pathogens enter the body from the outside, the more white blood cells need to be directed to the area. Leukotriene B4 is a central inflammatory mediator produced from arachidonic acid, and is a very potent chemoattractant that triggers the ‘invasion’ of white blood cells. It is produced by white blood cells themselves in an inflammatory environment, but we do not fully understand the regulation of leukotriene B4 release yet. This is one of the questions that could be resolved in the future by the method we have developed,” said Dr. Balázs Enyedi.
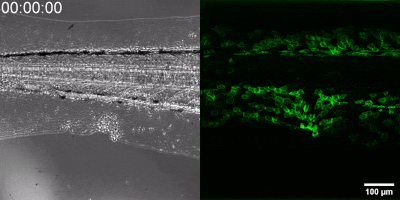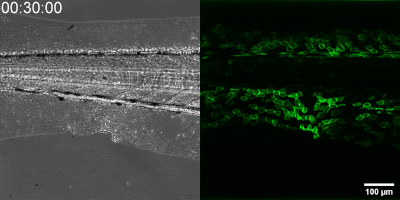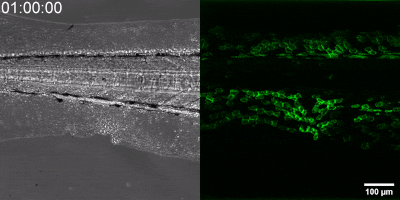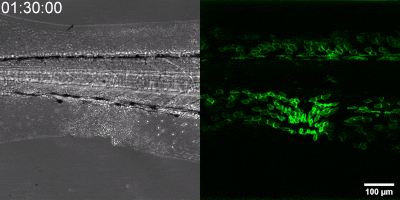 The new method they have developed is to create a biosensor starting from the leukotriene B4 receptor by modifying its sequence. The green fluorescence of the sensor is significantly increased by leukotriene B4, which can be measured under a fluorescence microscope, and the intensity of the green light can be seen to vary with the concentration of leukotriene B4 (as shown in the illustration). The DNA sequence encoding the biosensor can be delivered into the cells being tested in the experiments, which then generate the sensor themselves. This way, transgenic zebrafish or even mice can be created in which specific cells express the sensor, allowing microscopic detection of leukotriene B4 in their own environment. As a small molecule, leukotriene B4 diffuses away from the source in minutes, making it unassayable in fixed tissues by classical immunostaining methods. The new method, however, can be used to monitor the release of leukotriene B4 as a green fluorescent signal in transgenic animals in real time,” underlined Dr. Balázs Enyedi.
The new method they have developed is to create a biosensor starting from the leukotriene B4 receptor by modifying its sequence. The green fluorescence of the sensor is significantly increased by leukotriene B4, which can be measured under a fluorescence microscope, and the intensity of the green light can be seen to vary with the concentration of leukotriene B4 (as shown in the illustration). The DNA sequence encoding the biosensor can be delivered into the cells being tested in the experiments, which then generate the sensor themselves. This way, transgenic zebrafish or even mice can be created in which specific cells express the sensor, allowing microscopic detection of leukotriene B4 in their own environment. As a small molecule, leukotriene B4 diffuses away from the source in minutes, making it unassayable in fixed tissues by classical immunostaining methods. The new method, however, can be used to monitor the release of leukotriene B4 as a green fluorescent signal in transgenic animals in real time,” underlined Dr. Balázs Enyedi.
So far, the research group developed a biosensor for leukotriene B4, but their research could be generalized to develop sensors for additional molecules that regulate inflammatory processes.
 “The research has several forward-looking aspects. It will give us a better understanding of the biology and pathology of inflammation: there are many research teams working on this, whose dream for decades has been not only to understand the release and diffusion of molecules in principle, but also to be able to see this process. Our method will give us a better insight into the precise role of factors that regulate inflammation biology, even in different disease models – a key to developing more effective therapeutic tools in the future. On the other hand, the new chemoattractant biosensors that have been created and are currently under development also offer the possibility to find molecules and inhibitors that affect chemoattractant function more quickly and efficiently than current methods. Our current focus is to better understand the biological effects of inflammation-regulating molecules, and our longer-term plans include the identification of inhibitors to be used in pharmaceutical research,” said Dr. Balázs Enyedi.
“The research has several forward-looking aspects. It will give us a better understanding of the biology and pathology of inflammation: there are many research teams working on this, whose dream for decades has been not only to understand the release and diffusion of molecules in principle, but also to be able to see this process. Our method will give us a better insight into the precise role of factors that regulate inflammation biology, even in different disease models – a key to developing more effective therapeutic tools in the future. On the other hand, the new chemoattractant biosensors that have been created and are currently under development also offer the possibility to find molecules and inhibitors that affect chemoattractant function more quickly and efficiently than current methods. Our current focus is to better understand the biological effects of inflammation-regulating molecules, and our longer-term plans include the identification of inhibitors to be used in pharmaceutical research,” said Dr. Balázs Enyedi.
The researcher graduated from the Faculty of Medicine at Semmelweis University in 2006, and did his PhD at the Department of Physiology in the research group of Dr. Miklós Geiszt, professor at the institution, working on reactive oxygen derivatives. Already there, Dr. Balázs Enyedi developed fluorescence sensors to measure the levels of one of the reactive oxygen derivatives, hydrogen peroxide, in cells. He was subsequently recruited to the Institute of Cell Biology at Memorial Sloan Kettering Cancer Center in New York, USA. Here he was introduced to the zebrafish model system, among other things.
I was assigned to the laboratory of a young group leader, Dr. Philipp Niethammer, which was particularly fortunate regarding my career: as a postdoc, I was able to witness the construction of a laboratory and use this knowledge myself later on. I worked in the United States from 2011-2016, where I learned about the zebrafish model system, and then returned to Semmelweis University, where I started to create the infrastructure for zebrafish research and related live microscopy, which had not been established in the institution until then,
he pointed out. He received support not only from Semmelweis University: in 2018 he won the Hungarian Academy of Sciences’ Lendület Program, which, together with the university’s start-up program and the Innovation Directorate’s support programs, enabled him to start his research group at the Department of Physiology. “Whereas in the US I had a well-maintained, ready-made system, back home I had to start from scratch: from the meticulous architectural redesign of the animal house to the lighting system and the zebrafish habitat, everything had to be figured out with my team,” he explained, adding that during his years as a researcher in the US, he published papers in Nature Cell Biology, one of the most prestigious journals, as well as Cell, earning the trust to continue his research in Hungary. As a young working group leader, he was also selected as a grantee of the Hungarian Centre of Excellence in Molecular Medicine (HCEMM), where he is a member and has close links with the European Molecular Biology Laboratory in Heidelberg.
 The team’s findings were published in the prestigious journal Nature Communications, with an impact factor of 15-20. Although the paper was quickly approved by the journal, it took many years of work to get this far. “More important than the microscopy system I have built, the instrumentation and the zebrafish models, is the team I work with. There are currently ten of us: three PhD students, two MD-PhD students, two postdoctoral researchers and two laboratory and animal house managers, including French and German researchers. So the team is not only enthusiastic and young, but also international,” said Dr. Balázs Enyedi, adding that they are assisted by several TDK students, who are a pleasure to work with.
The team’s findings were published in the prestigious journal Nature Communications, with an impact factor of 15-20. Although the paper was quickly approved by the journal, it took many years of work to get this far. “More important than the microscopy system I have built, the instrumentation and the zebrafish models, is the team I work with. There are currently ten of us: three PhD students, two MD-PhD students, two postdoctoral researchers and two laboratory and animal house managers, including French and German researchers. So the team is not only enthusiastic and young, but also international,” said Dr. Balázs Enyedi, adding that they are assisted by several TDK students, who are a pleasure to work with.
Dr. Balázs Enyedi considers to be one of the most memorable moments on the way to publication when he saw their prototype leukotriene B4 sensor in action for the first time, a few days before Christmas one year ago. Then they had to work for nearly three years to successfully measure the release of leukotriene B4 from white blood cells in a zebrafish animal model. This was the main difficulty: based on scientific data, the release of leukotriene B4 was expected to occur in a different time window for many months, so it was long thought that there was a problem with the system because the measurements were performed for too short a time.
As the assistant professor said, their achievements have been congratulated by many people at national and international level. Before the publication in Nature Communications, he had already presented his research at several conferences, which attracted a lot of attention and interest – and this interest has just increased due to the publication in this prestigious journal. “This tool might be useful for many other scientists in their own experiments, so accordingly, several inquiries have been received from America to Germany and England,” he underlined. Dr. Balázs Enyedi added that the publication is only the beginning for his research team, which will set the path they want to follow in the next 5-10 years.
Ádám Szabó
Translation: Viktória Kiss
Photo: Attila Kovács – Semmelweis University