Seven COVID-19 patients have already been treated with the Hungarian remdesivir at Semmelweis University. Three clinics of the university participate in the clinical trial related to the remedy of coronavirus produced by Richter Gedeon Nyrt. At the same time, the American version of remdesivir has already been used at the university since the beginning of August: up until now it has been used in the treatment of 15 patients. Plasma therapy is part of the complex, high-quality therapy applied at the clinics, which has already been provided to 57 patients at Semmelweis University.
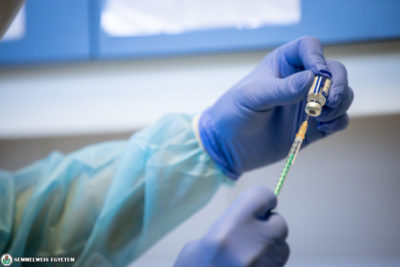
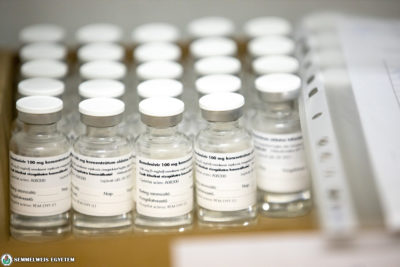 The anti-viral drug containing remdesivir, produced by Richter Gedeon Nyrt. was first used in Hungary on 14 October in the treatment of a patient at Semmelweis University’s Department of Pulmonology. The 75-year-old, female patient was in a serious condition, she arrived at the department for COVID-19 patients that day – said Dr. Veronika Müller, head of the department.
The anti-viral drug containing remdesivir, produced by Richter Gedeon Nyrt. was first used in Hungary on 14 October in the treatment of a patient at Semmelweis University’s Department of Pulmonology. The 75-year-old, female patient was in a serious condition, she arrived at the department for COVID-19 patients that day – said Dr. Veronika Müller, head of the department.
Currently altogether 7 patients are being treated with the Hungarian coronavirus drug and three clinics of the university participate in the related clinical trial: the Department of Pulmonology, the Department of Anesthesiology and Intensive Therapy, as well as the Department of Internal Medicine and Haematology.
At the same time, the American version of remdesivir has already been in use at the university as part of the standard therapy. At the Department of Anesthesiology and Intensive Therapy every COVID-19 patient, who is in need of a ventilator or oxygen-therapy, also receives remdesivir as part of their complex therapy. It was applied for the first time on 8 August 2020 – said Dr. János Gál, head of the department. Ever since 15 patients have been treated with this drug, typically for 5 days and occasionally for 10 days, at the three university clinics.
Remdesivir is an active agent that inhibits viral replication, it is administered to patients via an intravenous therapy. It can only be used in the treatment of those hospitalized patients, who are in a serious condition and are in need of oxygen therapy. However, in case of a serious hepatic impairment or a remarkable renal insufficiency this drug cannot be applied.
Plasma therapy is part of the complex, high-quality therapy applied in the treatment of COVID-19 patients at the clinics of Semmelweis University. Up until now 57 patients have been provided with the serum, which is prepared from the blood of individuals already recovered from coronavirus.
In addition, administering doses of dexamethasone is a mandatory element of the complex treatment protocol for ventilated patients since April, 2020. According to several studies, certain corticosteroids, like dexamethasone, can facilitate the success of the therapy with the reduction of the immune response. In full knowledge of this new evidence, dexamethasone is being used as a supplementary therapy at the university in case of COVID patients who are in need of oxygen.
The clinical trial related to remdesivir produced in Hungary started last week with the participation of all the four medical universities in Hungary.
Pálma Dobozi
Photo: Attila Kovács – Semmelweis University
Translation: Katalin Illés-Romhányi