The US Food and Drug Administration (FDA) has recently approved three high-sensitivity cardiac troponin assays (hs-cTn) for clinical use in the United States of America.
“There is no known concordance between the three assays. In our study we used all three hs-cTN assays to measure the troponin level in blood samples of patients with acute chest pain requiring emergency triage”, said dr. Júlia Karády, first author and PhD student at Semmelweis University.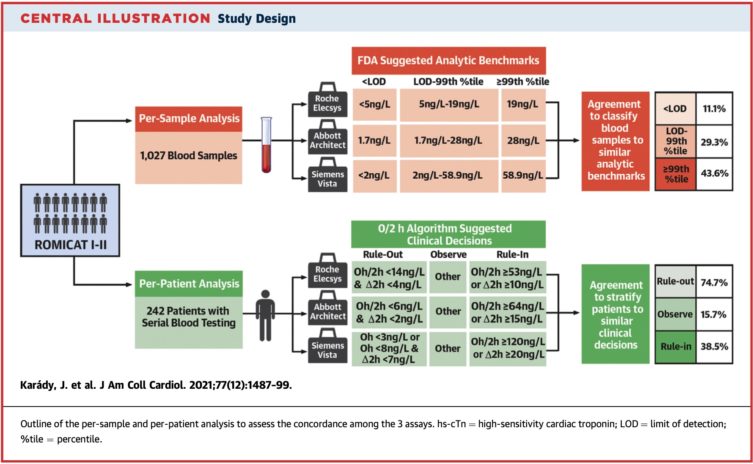
Based on the troponin level measurements taken at the same time, only 37.4% of all the blood samples were classified into the same analytical benchmark category by the three assays. In the subgroup analysis of 242 patients, the measurement of troponin levels was performed on serial samples and the three assays suggested the same clinical diagnosis in 74.8% of the cases.
It is important to draw the attention of clinicians to the significant differences between hs-cTn assays in clinical use, which is particularly important when patients are transferred from one hospital to the other and the two institutions use different types of hs-cTn assays.
Discordance of High-Sensitivity Troponin Assays in Patients WithSuspected Acute Coronary Syndromes
Júlia Karády MD, Thomas Mayrhofer PhD, Maros Ferencik MD, PhD, MCR, John T. Nagurney MD, MPH, James E. Udelson MD, Andreas A. Kammerlander MD, PhD, Jerome L. Fleg MD, W. Frank Peacock MD, James L. Januzzi MD, Wolfgang Koenig MD, PhD and Udo Hoffmann MD, MPH
JACC (Journal of the American College of Cardiology), 2021-03-30, Volume 77, Issue 12, Pages 1487-1499, Copyright © 2021 American College of Cardiology Foundation
10.1016/j.jacc.2021.01.046