1. 1. Effect of metabolic diseases on cardioprotective interventions and treatments against ischemia/reperfusion injury
Project supervisors: Prof. Dr. Peter Ferdinandy, Dr. Anikó Görbe, Dr. Zoltán Giricz
Project leaders: Dr. Tamás Gergely, Regina Nagy
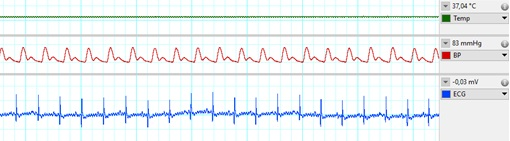
Metabolic derangements, such as obesity or diabetes, are major risk factors of cardiovascular diseases. The healthy heart can adapt to a certain level of ischemic injury (e.g., during a heart attack), but metabolic diseases, such as hypercholesterolemia, have a negative effect of this ischemia-tolerance of the heart, and may increase the extent of injury afflicted by ischemia/reperfusion. The mechanism of the changes in the myocardium due to metabolic co-morbidities are not fully understood, more detailed information on them would enable the development of novel cardioprotective therapies, which would lead to a better prognosis of ischemic heart diseases. In our Department infarction is inflicted on anesthetized high fat diet-fed rats, as a model for hypercholesterolemia, in our state of the art small animal surgery facility (Figure 1) by a surgical procedure, where the left descending coronary artery is occluded by placing a suture around it for 30-45 min then released. During surgery, we monitor vital parameters (e.g., blood pressure, ECG, temperature, respiration; Figure 2). Our undergraduate researchers learn surgical techniques and are involved actively in our ongoing studies.

Learning opportunities:
- literature search methods,
- designing in vivo rat studies
- working with metabolic disease models in rats,
- animal handling, oral treatment of small animals,
- performing cardiac surgeries on rats,
- performing quantitative PCR and Western blots,
- data evaluation and presentation.
Our major publications on the topic:
- Andreadou I, Daiber A, Baxter GF, Brizzi MF, Di Lisa F, Kaludercic N, Lazou A, Varga ZV, Zuurbier CJ, Schulz R, Ferdinandy P. Influence of cardiometabolic comorbidities on myocardial function, infarction, and cardioprotection: Role of cardiac redox signaling. Free Radic Biol Med. 2021 Apr;166:33-52.
- Andreadou I, Tsoumani M, Vilahur G, Ikonomidis I, Badimon L, Varga ZV, Ferdinandy P, Schulz R. PCSK9 in Myocardial Infarction and Cardioprotection: Importance of Lipid Metabolism and Inflammation. Front Physiol. 2020 Nov 12;11:602497. doi: 10.3389/fphys.2020.602497. PMID: 33262707; PMCID: PMC7688516.
- Andreadou I, Schulz R, Badimon L, Adameová A, Kleinbongard P, Lecour S, Nikolaou PE, Falcão-Pires I, Vilahur G, Woudberg N, Heusch G, Ferdinandy P. Hyperlipidaemia and cardioprotection: Animal models for translational studies. Br J Pharmacol. 2020 Dec;177(23):5287-5311
- Andreadou I, Iliodromitis EK, Lazou A, Görbe A, Giricz Z, Schulz R, Ferdinandy P. Effect of hypercholesterolemia on myocardial function, ischemia-reperfusion injury and cardioprotection by preconditioning, postconditioning and remote conditioning. Br J Pharmacol. 2017 Jun; 174 (12):1555-1569.
- Pavo N, Lukovic D, Zlabinger K, Zimba A, Lorant D, Goliasch G, Winkler J, Pils D, Auer K, Jan Ankersmit H, Giricz Z, Baranyai T, Sárközy M, Jakab A, Garamvölgyi R, Emmert MY, Hoerstrup SP, Hausenloy DJ, Ferdinandy P, Maurer G, Gyöngyösi M. Sequential activation of different pathway networks in ischemia-affected and non-affected myocardium, inducing intrinsic remote conditioning to prevent left ventricular remodeling. Sci Rep.2017 Mar 7;7:43958.
- Nagy CT, Koncsos G, Varga ZV, Baranyai T, Tuza S, Kassai F, Ernyey AJ, Gyertyán I, Király K, Oláh A, Radovits T, Merkely B, Bukosza N, Szénási G, Hamar P, Mathé D, Szigeti K, Pelyhe C, Jelemenský M, Onódi Z, Helyes Z, Schulz R, Giricz Z, Ferdinandy. Selegiline reduces adiposity induced by high-fat, high-sucrose diet in male rats. Br J Pharmacol. 2018 Sep;175(18):3713-3726.
- Koncsos G, Varga ZV, Baranyai T, Boengler K, Rohrbach S, Li L, Schlüter KD, Schreckenberg R, Radovits T, Oláh A, Mátyás C, Lux Á, Al-Khrasani M, Komlódi T, Bukosza N, Máthé D, Deres L, Barteková M, Rajtík T, Adameová A, Szigeti K, Hamar P, Helyes Z, Tretter L, Pacher P, Merkely B, Giricz Z, Schulz R, Ferdinandy P. Diastolic dysfunction in prediabetic male rats: Role of mitochondrial oxidative stress. Am J Physiol Heart Circ Physiol. 2016 Oct 1;311(4):H927-H943.
- Baranyai, T., et al., Acute hyperglycemia abolishes cardioprotection by remote ischemic perconditioning. Cardiovasc Diabetol, 2015. 14: p. 151.

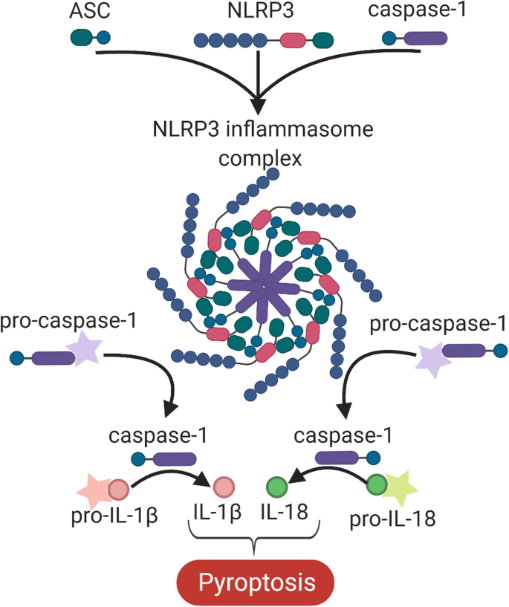
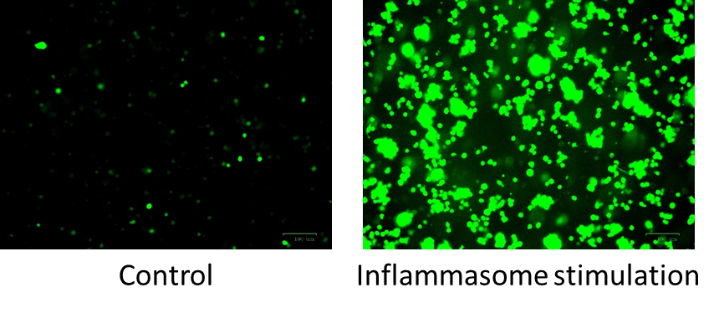
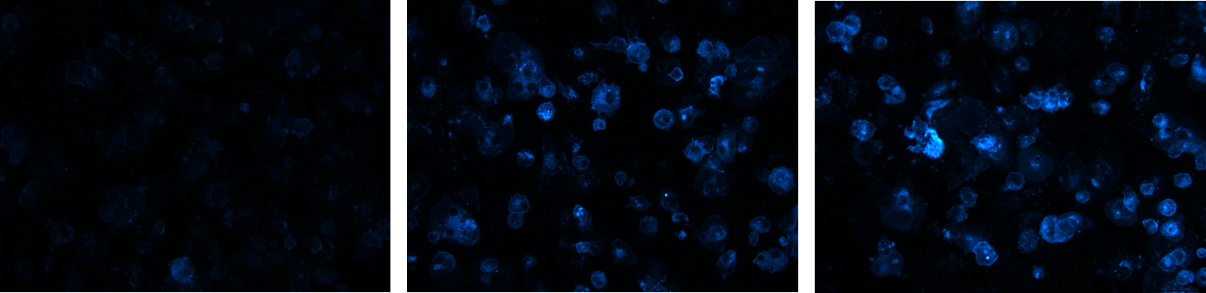
2. Effect of hypercholesterolemia on inflammasome activation in macrophages
Project supervisors: Dr. Anikó Görbe, Dr. Zoltán Varga
Project leader: Regina Nagy
Hypercholesterolemia induces cholesterol accumulation in immune cells, like macrophages, which promotes inflammation through Toll-like receptor (TLR) signaling and inflammasome activation. Intracellular cholesterol accumulation and the consequent inflammatory response is probably beneficial in the response to infection, however, it worsens diseases in association with chronic inflammation, like atherosclerosis. Inflammation also plays a critical role in the genesis, progression, and manifestation of several cardiovascular diseases. Direct inhibition of inflammasomes, rather than proinflammatory cytokine suppression, can be an effective anti-inflammatory management. Thus, exploring the molecular mechanisms behind inflammasome activation can lead to the discovery of new potential drug targets in hypercholesterolemic cardiovascular comorbidities. In our project we aim to investigate the effect of in vitro hypercholesterolemic treatment on the inflammatory mechanisms of human monocytes/macrophages.
Learning opportunities:
- literature search methods,
- working with metabolic disease models in cell cultures,
- designing in vitro cell culture experiments,
- maintaining cell cultures,
- treatment of cells with pharmacological compounds,
- performing quantitative PCR and Western blots,
- data evaluation and presentation.