The acute cessation of blood supply to the cardiac musculature during infarction leads to irreversible tissue damage and necrosis. These days the most efficient therapy to save the myocardium is the revascularization by either thrombolysis, percutaneous coronary intervention (PCI), or coronary bypass (CABG) surgery. However, the restoration of blood flow leads to further tissue damage. This phenomenon is termed ischemia/reperfusion injury, which can manifest in 4 ways: an increase in the amount of infarcted tissues (see Figure 1), more pronounced microvascular obstruction, increased probability of arrhythmias, decreased cardiac contractility.
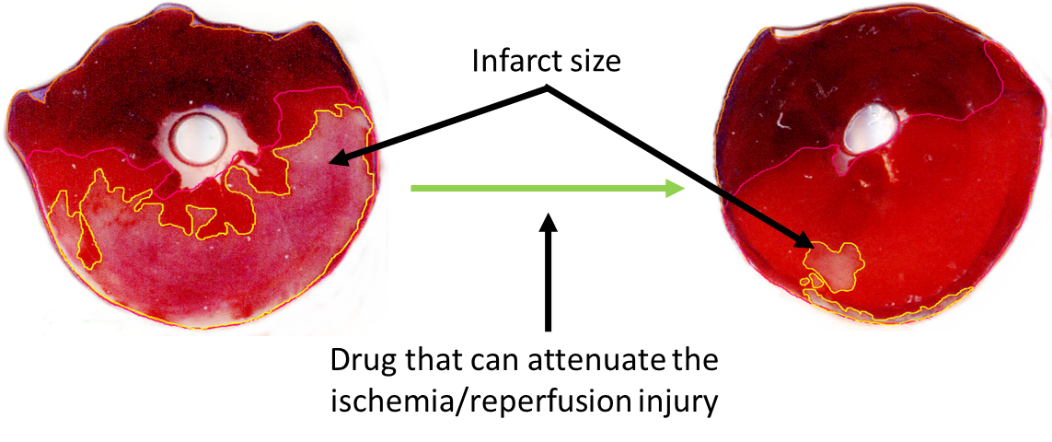
Licensed pharmacological tool for the treatment of ischemia/reperfusion injury is not yet available despite the very intensive research in the field, however, there is a significant need for them to improve acute and long-term survival and/or quality of life of patients with infarction. Therefore, we study the molecular aspects of cardiac ischemia/reperfusion injury to identify novel pharmacological targets. Furthermore, we study the interaction between pharmacons used for other symptoms and the extent of ischemia/reperfusion injury (hidden cardiotoxicity of drugs), and the effect of metabolic diseases on cardioprotective interventions and treatments.
1. Effect of pharmacological agents on ischemia/reperfusion injury in small animal models: the significance of hidden cardiotoxicity
Project supervisors: Dr. Anikó Görbe, Dr. Zoltán Giricz, Prof. Dr. Péter Ferdinandy
Project leaders: Dr. Tamás Gergely, Bennet Weber, Regina Nagy
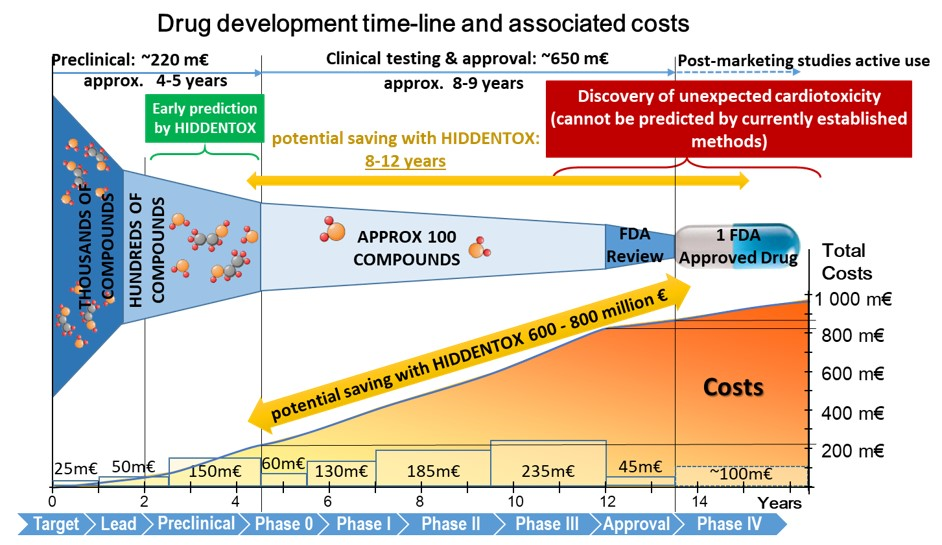
Unexpected cardiac adverse effects are the leading causes of discontinuation of clinical trials and withdrawal of drugs from the market (see Figure 2). Since the original observations in the mid-90s, it has been well established that cardiovascular risk factors and comorbidities (such as ageing, hyperlipidaemia, and diabetes) and their medications (e.g. nitrate tolerance, adenosine triphosphate-dependent potassium inhibitor antidiabetic drugs, statins, etc.) may interfere with cardiac ischemic tolerance and endogenous cardioprotective signaling pathways. Indeed, drugs may exert unwanted effects on the diseased and treated heart that is hidden in the healthy myocardium. Hidden cardiotoxic effects may be due to (i) drug-induced enhancement of deleterious signaling due to ischemia/reperfusion injury and/or the presence of risk factors and/or (ii) inhibition of cardioprotective survival signaling pathways, both of which may lead to ischemia-related cell death and/or pro-arrhythmic effects. This led to a novel concept of ‘hidden cardiotoxicity’, defined as cardiotoxicity of a drug that manifests only in the diseased heart with e.g. ischemia/reperfusion injury and/or in the presence of its major comorbidities. Little is known on the mechanism of hidden cardiotoxicity, moreover, hidden cardiotoxicity cannot be revealed by the routinely used non-clinical cardiac safety testing methods on healthy animals or tissues. Therefore, here, we emphasize the need for development of novel cardiac safety testing platform involving combined experimental in vivo and in vitro models of cardiac diseases (especially myocardial ischemia/reperfusion and ischemic conditioning) in the presence and absence of major cardiovascular comorbidities and/or cotreatments. (Ferdinandy et al, European heart journal, 2018).
Learning opportunities:
- literature search methods,
- designing in vivo rat studies,
- animal handling, oral treatment of small animals,
- performing cardiac surgeries on rats,
- performing quantitative PCR and Western blots,
- data evaluation and presentation.
Our major publications on the topic:
Ferdinandy, P. et al. Definition of hidden drug cardiotoxicity: paradigm change in cardiac safety testing and its clinical implications. Eur Heart J, 2018.
Ferdinandy, P., et al. Interaction of risk factors, comorbidities, and comedications with ischemia/reperfusion injury and cardioprotection by preconditioning, postconditioning, and remote conditioning. Pharmacol Rev, 2014. 66(4): p. 1142-74.
Brenner GB., et al. Hidden Cardiotoxicity of Rofecoxib Can be Revealed in Experimental Models of Ischemia/Reperfusion. Cells. 2020 Feb 26;9(3):551.
Weber BY, et al. Rosiglitazone Does Not Show Major Hidden Cardiotoxicity in Models of Ischemia/Reperfusion but Abolishes Ischemic Preconditioning-Induced Antiarrhythmic Effects in Rats In Vivo. Pharmaceuticals (Basel). 2022 Aug 26;15(9):1055.
Gergely TG, et al. Effects of Bempedoic Acid in Acute Myocardial Infarction in Rats: No Cardioprotection and No Hidden Cardiotoxicity. Int J Mol Sci. 2023 Jan 13;24(2):1585.
2. Effect of pharmacological agents on ischemia/reperfusion injury in in vitro cell culture models
Project supervisor: Dr. Anikó Görbe
Project leaders: Bennet Weber, Regina Nagy
The hidden cardiotoxic effect of pharmacological compounds can be further tested in in vitro cell cultures. We have previously demonstrated the potential to investigate the safety of drug candidates in in vitro cell culture models in which simulation of ischemia/reperfusion (I/R) damage, and comorbidities can be achieved. Cardiac cell lines and primary isolated cardiac cells can be used for high throughput drug screening and their use is beneficial to assess the direct effects of drugs on cardiac cell types. In our project we test different agents that might show direct cardiotoxic effect on cardiac cells only in the presence of comorbidities like hypercholesterolemia or I/R injury.

earning opportunities:
- literature search methods,
- designing in vitro cell culture experiments,
- isolation of primary cardiac cells,
- maintaining cell cultures,
- treatment of cells with pharmacological compounds,
- performing viability assays,
- data evaluation and presentation.
Our major publications on the topic:
Brenner GB., et al. Hidden Cardiotoxicity of Rofecoxib Can be Revealed in Experimental Models of Ischemia/Reperfusion. Cells. 2020 Feb 26;9(3):551. (link)
Weber BY, et al. Rosiglitazone Does Not Show Major Hidden Cardiotoxicity in Models of Ischemia/Reperfusion but Abolishes Ischemic Preconditioning-Induced Antiarrhythmic Effects in Rats In Vivo. Pharmaceuticals (Basel). 2022 Aug 26;15(9):1055. (link)
3. Investigation of cardiac transcriptomic changes induced by pharmacological agents showing hidden cardiac effects
Project supervisor: Dr. Anikó Görbe
Project leaders: Bennet Weber, Regina Nagy
Endogenous cardioprotective processes and molecules are well known in the reduction of myocardial ischemia / reperfusion (I/R) damage, the effects of which are often lost during clinical translation, especially in the presence of comorbidities. In addition, many drugs may show myocardial side effects / hidden cardiotoxicity that have not been elucidated in current drug development practices. Changes in gene expression may also play a role in hidden cardiotoxicity, but these have not been fully explored and their research is a new aspect of safety pharmacology. In our project we aim to investigate the expression profile of myocardial microRNA and mRNA in rat treated with a drug with proven cardiotoxicity in an acute I/R model may result in a number of differentially expressed target molecules. Based on the revealed expression profile changes, the mechanisms responsible for the side effects can be identified. The potential toxic or protective effects of selected microRNAs can be validated in an I/R system in a human cardiomyocyte model. Molecular targets predicted in bioinformatics analysis can also be validated at transcript and protein levels.
Learning opportunities:
- literature search methods,
- designing in vitro cell culture experiments,
- maintaining cell cultures,
- treatment of cells with pharmacological compounds,
- miRNA target search and validation by quantitative PCR and Western blots,
- data evaluation and presentation.
Our major publications on the topic:
Sághy É, et al. Cardiac miRNA Expression and their mRNA Targets in a Rat Model of Prediabetes. Int J Mol Sci. 2020 Mar 20;21(6):2128.





