Project supervisors:


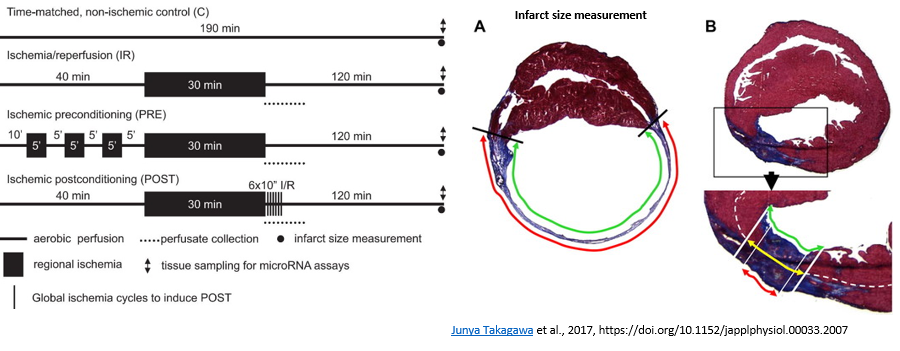
 Ischemic heart diseases and their pathological consequences belong to the leading causes of death worldwide. In the acute treatment of myocardial ischemia one of the most important tasks is the restoration of the tissue perfusion.
Ischemic heart diseases and their pathological consequences belong to the leading causes of death worldwide. In the acute treatment of myocardial ischemia one of the most important tasks is the restoration of the tissue perfusion.
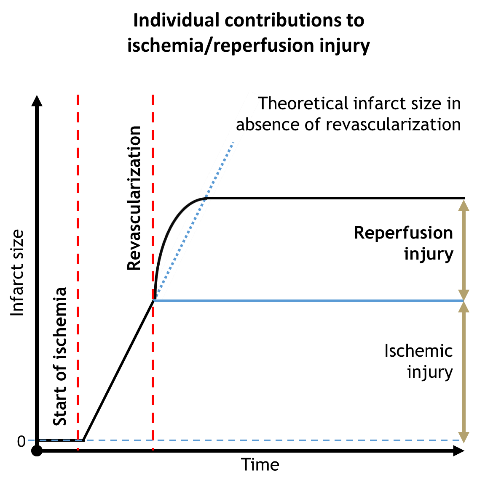
Unfortunately, reperfusion also causes further damage to the myocardium which is usually referred as reperfusion injury. In certain cases it is responsible for half of the total myocardial damage.
Despite that several small molecule drugs were found to decrease the infarct size in preclinical settings none of those were successfully translated to the human therapy. Therefore, it is necessary to find novel approaches and effective cardioprotective candidate molecules for future drug development.
In the recent decades the potential application of oligonucleotide molecules such as miRNAs has emerged as an attractive and innovative approach in the treatment of various pathologies including cancers and even cardiovascular diseases.
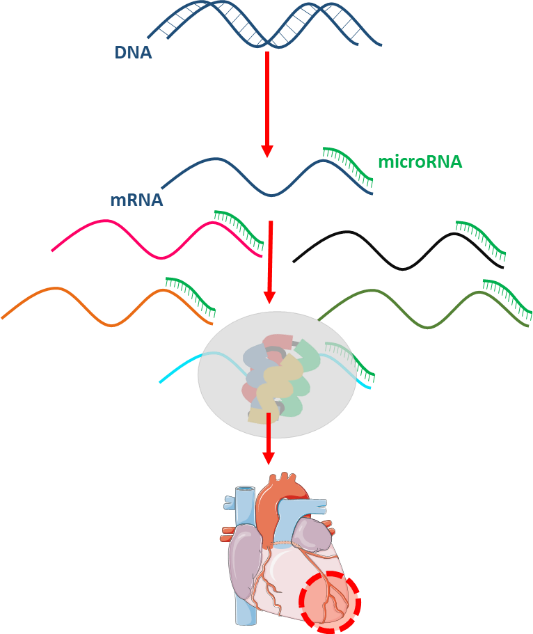 It was identified that the dysregulation of miRNAs plays an important role in the pathomechanism myocardial infarction. MicroRNAs are short, ~18-25 nucleotide-long non-coding RNA sequences those negatively regulate gene expression at the post-transcriptional level either by inhibition of the translation or promotion of the target mRNA degradation. Even entire cellular pathways can be regulated by a single microRNA, therefore miRNAs have a great potential to become multi-target drugs for diseases with multifactorial origin. Two distinct types of the microRNA therapies are microRNA mimics and antagomiRs (Varga ZV et al., Am J Physiol Heart Circ Physiol, 10.1152/ajpheart.00812.2013). MicroRNA mimics are double-stranded sequences, including “guide” strand, having the same sequence as its endogenous counterpart, and a not fully complementary passenger strand, which degrades after the detachment. AntagomiRs are essentially single-stranded antisense oligonucleotides with complementarity to an endogenous miRNA thereby blocking its function.
It was identified that the dysregulation of miRNAs plays an important role in the pathomechanism myocardial infarction. MicroRNAs are short, ~18-25 nucleotide-long non-coding RNA sequences those negatively regulate gene expression at the post-transcriptional level either by inhibition of the translation or promotion of the target mRNA degradation. Even entire cellular pathways can be regulated by a single microRNA, therefore miRNAs have a great potential to become multi-target drugs for diseases with multifactorial origin. Two distinct types of the microRNA therapies are microRNA mimics and antagomiRs (Varga ZV et al., Am J Physiol Heart Circ Physiol, 10.1152/ajpheart.00812.2013). MicroRNA mimics are double-stranded sequences, including “guide” strand, having the same sequence as its endogenous counterpart, and a not fully complementary passenger strand, which degrades after the detachment. AntagomiRs are essentially single-stranded antisense oligonucleotides with complementarity to an endogenous miRNA thereby blocking its function.
1. Explorative pharmacokinetical profiling of miRNAs in murine models
Project leader and contact person:

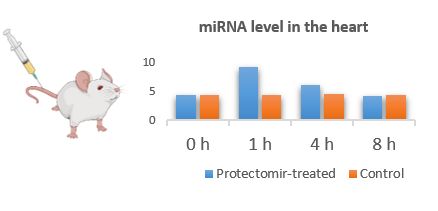 Although, the application of miRNAs are actively investigated in various indications, little is known about their pharmacokinetical properties and the differences in the characteristics between various miRNAs. Therefore, we aim to explore the pharmacokinetic profile of miRNA mimics and AntagomiRs in healthy animals.
Although, the application of miRNAs are actively investigated in various indications, little is known about their pharmacokinetical properties and the differences in the characteristics between various miRNAs. Therefore, we aim to explore the pharmacokinetic profile of miRNA mimics and AntagomiRs in healthy animals.
Learning opportunities:
- In vivo experimental methodologies (e.g., treatment with miRNAs, plasma/tissue sampling)
- In vitro methods (e.g., western blot, RNA isolation, qRT-PCR)
- Histological methods (e.g., fixation, sectioning, staining methods, RNAscope)
2. In vivo proof of concept studies of protectomiRs
Project leader and contact person: Dr. András Makkos
In these studies, we aim to explore and prove the cardioprotective effect of novel or previously identified protectomiRs (miRNAs) in murine model of myocardial infarction and heart failure. In addition, we also aim to characterize the tissue specific target gene expression profile changes both in the heart and various tissue types.
Learning opportunities:
- In vivo experimental methodologies (treatment with miRNAs, plasma/tissue sampling, murine models of myocardial infarction or heart failure)
- Histological methods (e.g. fixation, sectioning, staining methods, RNAscope)
- In vitro methodologies (e.g. western blot, RNA isolation, qRT-PCR)
3. Development of chemically modified miRNA mimics/AntagomiRs
Project leader and contact person:

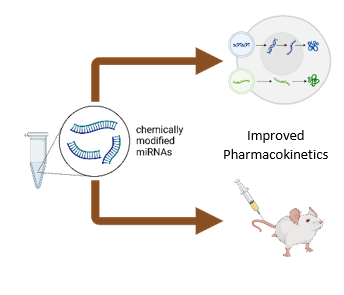 During the development of miRNA-based pharmacotherapies it is a crucial question to provide the proper bioavailability and tissue specificity meanwhile minimizing the potential toxicity of the medication. Accordingly, we are actively working on chemical modification-based improvement of our patented cardioprotective miRNA sequences in order to improve their pharmacokinetic properties and safety.
During the development of miRNA-based pharmacotherapies it is a crucial question to provide the proper bioavailability and tissue specificity meanwhile minimizing the potential toxicity of the medication. Accordingly, we are actively working on chemical modification-based improvement of our patented cardioprotective miRNA sequences in order to improve their pharmacokinetic properties and safety.
Learning opportunities:
- In vivo experimental methodologies (treatment with miRNAs, plasma/tissue sampling)
- in silico modelling, preparative chemistry
- Cell culturing, cell culture-based experimental models (e.g. miRNA transfection, simulated-ischemia reperfusion model, viability assays, fluorescence signal measurement)
- Confocal microscopy imaging
- Histological methods (e.g. fixation, sectioning, staining methods, RNAscope)
- In vitro methodologies (e.g. western blot, RNA isolation, qRT-PCR)
4. Development of novel vehicle formulations with miRNA mimics/AntagomiRs
Project leader and contact person: Dr. Imre Vörös
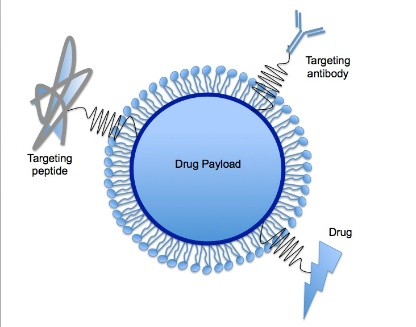 As it was mentioned earlier the proper tissue specificity and plasma/tissue half-life of the oligonucleotide-based therapies are challenging to provide. In addition to the chemical modifications the application of innovative vehicles could greatly improve the pharmacokinetic properties of cardioprotective miRNAs (e.g. tissue specifity, cellular uptake)
As it was mentioned earlier the proper tissue specificity and plasma/tissue half-life of the oligonucleotide-based therapies are challenging to provide. In addition to the chemical modifications the application of innovative vehicles could greatly improve the pharmacokinetic properties of cardioprotective miRNAs (e.g. tissue specifity, cellular uptake)
Learning opportunities:
- Cell culturing, cell culture-based experimental models (e.g. miRNA transfection, simulated-ischemia reperfusion model, viability assays, fluorescence signal measurement)
- Confocal microscopy imaging
- In vivo experimental methodologies (treatment with oligonucleotides, plasma/tissue sampling, murine models of myocardial infarction or heart failure)
- Histological methods (e.g. fixation, sectioning, staining methods, RNAscope)
Publications:
- Makkos, A., Ágg, B., Petrovich, B., Varga, Z. V., Görbe, A., & Ferdinandy, P. (2021). Systematic review and network analysis of microRNAs involved in cardioprotection against myocardial ischemia/reperfusion injury and infarction: Involvement of redox signalling. Free radical biology & medicine, 172, 237–251. https://doi.org/10.1016/j.freeradbiomed.2021.04.034
- Makkos, A., Ágg, B., Varga, Z. V., Giricz, Z., Gyöngyösi, M., Lukovic, D., Schulz, R., Barteková, M., Görbe, A., & Ferdinandy, P. (2021). Molecular Network Approach Reveals Rictoras a Central Target of Cardiac ProtectomiRs. International journal of molecular sciences, 22(17), 9539. https://doi.org/10.3390/ijms22179539
- Varga, Z. V., Zvara, A., Faragó, N., Kocsis, G. F., Pipicz, M., Gáspár, R., Bencsik, P., Görbe, A., Csonka, C., Puskás, L. G., Thum, T., Csont, T., & Ferdinandy, P. (2014). MicroRNAs associated with ischemia-reperfusion injury and cardioprotection by ischemic pre- and postconditioning: protectomiRs. American journal of physiology. Heart and circulatory physiology, 307(2), H216–H227. https://doi.org/10.1152/ajpheart.00812.2013
- Ferdinandy, P., Andreadou, I., Baxter, G. F., Bøtker, H. E., Davidson, S. M., Dobrev, D., Gersh, B. J., Heusch, G., Lecour, S., Ruiz-Meana, M., Zuurbier, C. J., Hausenloy, D. J., & Schulz, R. (2023). Interaction of Cardiovascular Nonmodifiable Risk Factors, Comorbidities and Comedications With Ischemia/Reperfusion Injury and Cardioprotection by Pharmacological Treatments and Ischemic Conditioning. Pharmacological reviews, 75(1), 159–216. https://doi.org/10.1124/pharmrev.121.000348
- Onódi, Z., Visnovitz, T., Kiss, B., Hambalkó, S., Koncz, A., Ágg, B., Váradi, B., Tóth, V. É., Nagy, R. N., Gergely, T. G., Gergő, D., Makkos, A., Pelyhe, C., Varga, N., Reé, D., Apáti, Á., Leszek, P., Kovács, T., Nagy, N., Ferdinandy, P., … Varga, Z. V. (2022). Systematic transcriptomic and phenotypic characterization of human and murine cardiac myocyte cell lines and primary cardiomyocytes reveals serious limitations and low resemblances to adult cardiac phenotype. Journal of molecular and cellular cardiology, 165, 19–30. https://doi.org/10.1016/j.yjmcc.2021.12.007
- Schreckenberg, R., Klein, J., Kutsche, H. S., Schulz, R., Gömöri, K., Bencsik, P., Benczik, B., Ágg, B., Sághy, É., Ferdinandy, P., & Schlüter, K. D. (2020). Ischaemic post-conditioning in rats: Responder and non-responder differ in transcriptome of mitochondrial proteins. Journal of cellular and molecular medicine, 24(10), 5528–5541. https://doi.org/10.1111/jcmm.15209
- Tamargo, J., Agewall, S., Borghi, C., Ceconi, C., Cerbai, E., Dan, G. A., Ferdinandy, P., Grove, E. L., Rocca, B., Sulzgruber, P., Semb, A. G., Sossalla, S., Niessner, A., Kaski, J. C., & Dobrev, D. (2023). New pharmacological agents and novel cardiovascular pharmacotherapy strategies in 2022. European heart journal. Cardiovascular pharmacotherapy, 9(4), 353–370. Advance online publication. https://doi.org/10.1093/ehjcvp/pvad034
- Weber, B. Y., Brenner, G. B., Kiss, B., Gergely, T. G., Sayour, N. V., Tian, H., Makkos, A., Görbe, A., Ferdinandy, P., & Giricz, Z. (2022). Rosiglitazone Does Not Show Major Hidden Cardiotoxicity in Models of Ischemia/Reperfusion but Abolishes Ischemic Preconditioning-Induced Antiarrhythmic Effects in Rats In Vivo. Pharmaceuticals (Basel, Switzerland), 15(9), 1055. https://doi.org/10.3390/ph15091055
- Sághy, É., Vörös, I., Ágg, B., Kiss, B., Koncsos, G., Varga, Z. V., Görbe, A., Giricz, Z., Schulz, R., & Ferdinandy, P. (2020). Cardiac miRNA Expression and their mRNA Targets in a Rat Model of Prediabetes. International journal of molecular sciences, 21(6), 2128. https://doi.org/10.3390/ijms21062128
- Vörös, I., Sághy, É., Pohóczky, K., Makkos, A., Onódi, Z., Brenner, G. B., Baranyai, T., Ágg, B., Váradi, B., Kemény, Á., Leszek, P., Görbe, A., Varga, Z. V., Giricz, Z., Schulz, R., Helyes, Z., & Ferdinandy, P. (2021). Somatostatin and Its Receptors in Myocardial Ischemia/Reperfusion Injury and Cardioprotection. Frontiers in pharmacology, 12, 663655. https://doi.org/10.3389/fphar.2021.663655
- Brenner, G. B., Makkos, A., Nagy, C. T., Onódi, Z., Sayour, N. V., Gergely, T. G., Kiss, B., Görbe, A., Sághy, É., Zádori, Z. S., Lázár, B., Baranyai, T., Varga, R. S., Husti, Z., Varró, A., Tóthfalusi, L., Schulz, R., Baczkó, I., Giricz, Z., & Ferdinandy, P. (2020). Hidden Cardiotoxicity of Rofecoxib Can be Revealed in Experimental Models of Ischemia/Reperfusion. Cells, 9(3), 551. https://doi.org/10.3390/cells9030551
- Spannbauer, A., Traxler, D., Lukovic, D., Zlabinger, K., Winkler, J., Gugerell, A., Ferdinandy, P., Hausenloy, D. J., Pavo, N., Emmert, M. Y., Hoerstrup, S. P., Jakab, A., Gyöngyösi, M., & Riesenhuber, M. (2019). Effect of Ischemic Preconditioning and Postconditioning on Exosome-Rich Fraction microRNA Levels, in Relation with Electrophysiological Parameters and Ventricular Arrhythmia in Experimental Closed-Chest Reperfused Myocardial Infarction. International journal of molecular sciences, 20(9), 2140. https://doi.org/10.3390/ijms20092140
- Sayour, N. V., Brenner, G. B., Makkos, A., Kiss, B., Kovácsházi, C., Gergely, T. G., Aukrust, S. G., Tian, H., Zenkl, V., Gömöri, K., Szabados, T., Bencsik, P., Heinen, A., Schulz, R., Baxter, G. F., Zuurbier, C. J., Vokó, Z., Ferdinandy, P., & Giricz, Z. (2023). Cardioprotective efficacy of limb remote ischaemic preconditioning in rats: discrepancy between a meta-analysis and a three-centre in vivo study. Cardiovascular research, 119(6), 1336–1351. https://doi.org/10.1093/cvr/cvad024
- Schreckenberg, R., Wolf, A., Szabados, T., Gömöri, K., Szabó, I. A., Ágoston, G., Brenner, G., Bencsik, P., Ferdinandy, P., Schulz, R., & Schlüter, K. D. (2022). Proprotein Convertase Subtilisin Kexin Type 9 (PCSK9) Deletion but Not Inhibition of Extracellular PCSK9 Reduces Infarct Sizes Ex Vivo but Not In Vivo. International journal of molecular sciences, 23(12), 6512. https://doi.org/10.3390/ijms23126512