The core interest of our workgroup is to uncover novel roles of EVs in cardiac diseases, to assess their prognostic, diagnostic and therapeutical potential, and to develop novel technologies in EV research.
Goals and opportunities for our undergrad students:
- Participation in drug development and pharmacology research
- Learning opportunity on how to design, analyze and present scientific results
- Usage of cutting-edge technologies in our in vitro-, in vivo-, and cell culture labs.
- Possibility to broaden your scientific expertise and to enhance your presentation skills in our weekly science club (2 additional credit points – https://semmelweis.hu/pharmacology/en/education/elective-course/)
- Personalized mentoring to provide the necessary support to excel at the annual Semmelweis-TDK student conference and the national OTDK conference, and for the most active students, even international conferences.
- Thesis based on your own research work, rector´s competition for selected topics.
- Co-authorship on scientific publications for the most devoted active students.
- Embrace your dedication to research and embark on an extraordinary journey toward your dreams of pursuing a PhD. Seize the opportunity to start your MD-PhD or Conventional PhD with us and pave the way to a future filled with endless possibilities.
Major research topics:
- Role of extracellular vesicles in cardiovascular diseases and its comorbidities
We are to understand how EVs induce and affect cardiovascular diseases. To this end, we investigate its changes in disease models of the cardiovascular system and in its comorbidities, e.g., metabolic diseases. We analyze the change of EV characteristics in preclinical animal and cell culture models as well as in clinical samples. We believe that a better understanding on the role of EVs in the pathophysiology of these diseases will lead to novel diagnostic and therapeutic solutions. (10.1093/cvr/cvx211)
In this project, we seek for undergraduate- and PhD students and Post-doc researchers who are interested in molecular biology of the heart and basic science.
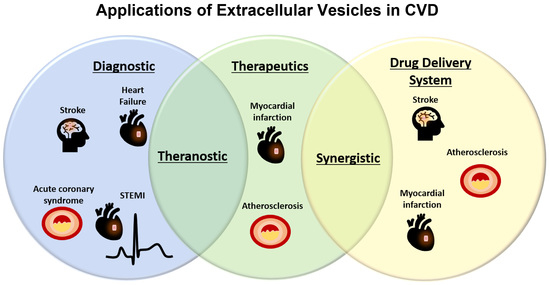
- Extracellular vesicles in regenerative medicine: potential in the failing heart
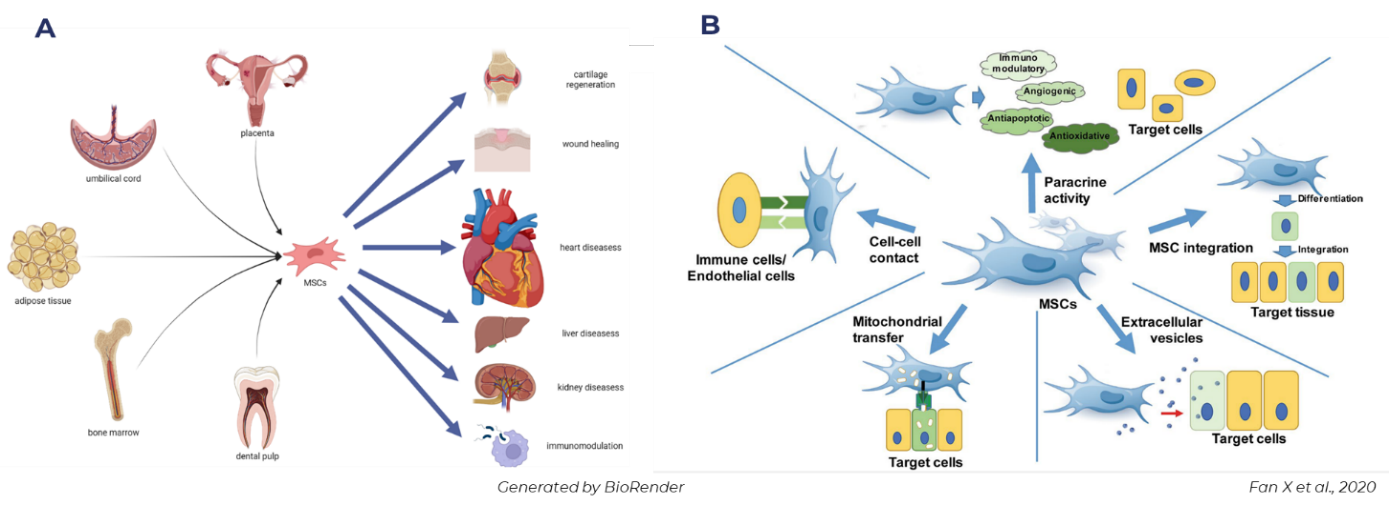
Extracellular vesicles (EVs) represent a promising therapeutic option for the prevention and treatment cardiovascular diseases (10.3390/ijms21207687). Our primary objective is to establish EV-based therapies that are well-suited for addressing heart failure and other cardiovascular conditions. For this purpose, we investigate EVs derived from mesenchymal stem cells (MSCs) which have shown promising regenerative results (10.3390/ijms23126480). We also develop relevant in vitro and cell-based assays for the accurate quantification of the regenerative efficacy of various EV isolates.
In this project, we seek for undergraduate students who are interested in molecular biology of the heart and basic science. Students will be involved in planning and implementation of research projects, including the design of preclinical models, with the utilization of state-of-the-art technology, and data analyses.
- Development of novel technologies and tools for the isolation of high-purity EVs
We have developed methods for the isolation of highly pure EVs from biofluids. These are based on ultracentrifugation followed by size exclusion chromatography (10.1371/journal.pone.0145686) or density gradient ultracentrifugation coupled with bind-elute size exclusion chromatography. This project aims to improve these approaches to eliminate impurities from blood plasma-derived EV preparations. In parallel, together with Pharmahungary, we run an engineering program to develop the equipment that utilizes our isolation technology.
In this project, we seek for students who are interested in analytical technologies, integrated software and hardware engineering or method-and product development.
Contact:
- Lab: Budapest, Hungary, H-1085, Nagyvárad tér 4., 4th floor; Department of Pharmacology & Pharmacotherapy, Semmelweis University
- Email: giricz.zoltan@semmelweis.hu
- Phone: +3612104416
Our selected publications:
