Projects
Understanding Immunotherapy resistance and response of small cell lung cancer
Project leaders: Ágnes Paál, Anikó Görbe
 |
 |
Small cell lung cancer (SCLC) is one of the malignancies with the worst prognosis, for which there have been no significant advances in therapy over the last three decades. The first promising results have been with the use of immune checkpoint inhibitors (ICIs), but success is far below that seen in non-small cell lung cancer, where the use of ICIs has been a breakthrough in therapy. In SCLC, in some patients with extensive-stage disease, survival can be 1-2 months longer.
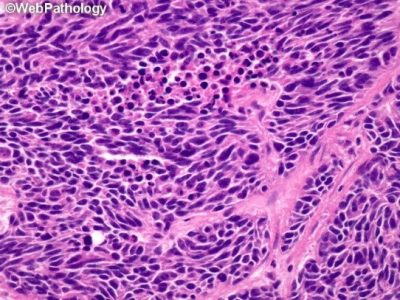
Microscopical image of small round cells in small cell lung cancer
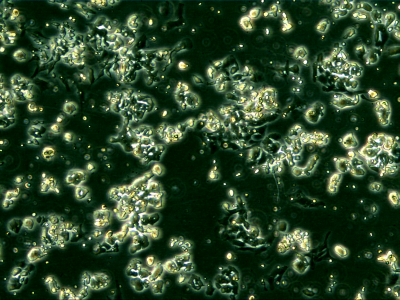
SCLC cells in culture
These suggest that new approaches are needed to understand response and resistance of SCLC to Immunotherapy. Several research groups have identified molecular subtypes of SCLC, which partially correspond to each other. For example, weaker neuroendocrine marker expression is often associated with stronger immune infiltration (neuroendocrine-low, immune oasis tumors), which may suggest better Immunotherapy responsiveness in such tumors.
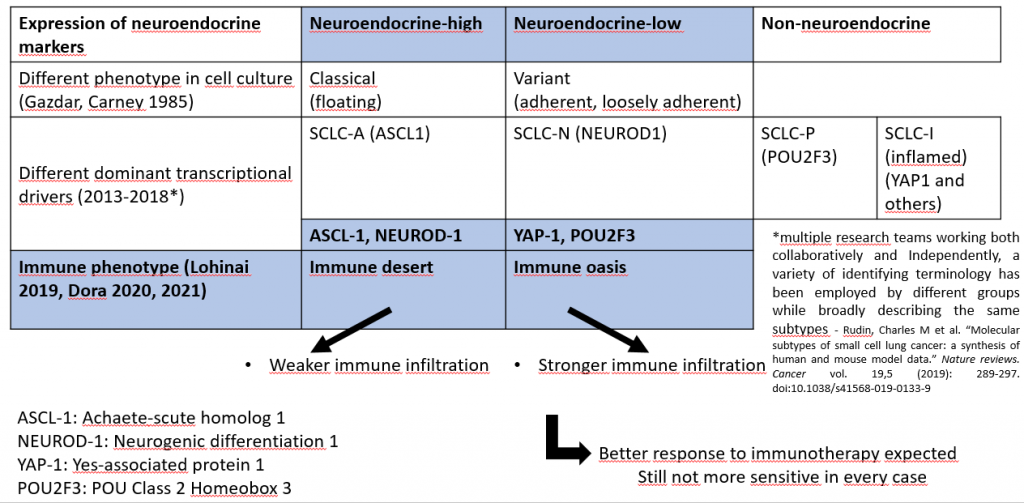
A possible road to improved therapy: evolving subclassificiation of SCLC
Despite stronger immune infiltration, tumors do not always respond to ICIs, and local overexpression of immunosuppressive molecules has been detected.
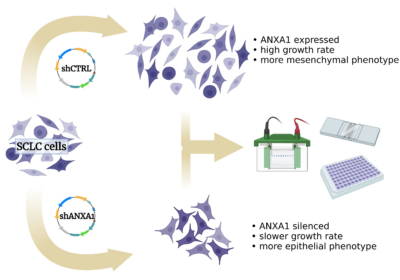
We investigate the effects of silencing gene expression of one of these immunosuppressive molecules, annexin A1, in cell culture of neuroendocrine-low SCLC cell lines. We hypothesize that some features of the malignant phenotype may be affected by annexin A1 silencing, such as growth rate, epithelio-mesenchymal transition, metabolic shift, dominant transcription drivers and sensitivity to the classical chemotherapeutic agents, cisplatin and etoposide.
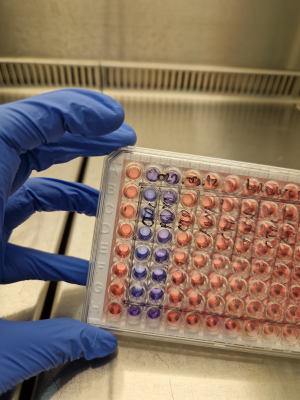
Fluorescent viability assay
We believe that studying the effects of annexin A1 silencing may help understand new aspects of small cell lung cancer that are relevant in therapy.
Learning opportunities:
- culturing of small cell lung cancer cell lines
- assessment of growth rate by cell counting and fluorescence viability assays
- treatment of cells and chemosensitivity tests with fluorescence viability assays
- western blot
Related publications:
-
Dora, David et al. “Neuroendocrine subtypes of small cell lung cancer differ in terms of immune microenvironment and checkpoint molecule distribution.” Molecular oncology vol. 14,9 (2020): 1947-1965. doi:10.1002/1878-0261.12741
-
Dora, David et al. “Characterization of Tumor-Associated Macrophages and the Immune Microenvironment in Limited-Stage Neuroendocrine-High and -Low Small Cell Lung Cancer.” Biology vol. 10,6 502. 4 Jun. 2021, doi:10.3390/biology10060502
-
Dora, David et al. “Protein Expression of immune checkpoints STING and MHCII in small cell lung cancer.” Cancer immunology, Immunotherapy : CII vol. 72,3 (2023): 561-578. doi:10.1007/s00262-022-03270-w
Development of miRNAs as a new therapeutic tool in the treatment of cardiovascular diseases
Project supervisors: Prof. Dr. Péter Ferdinandy Dr. Anikó Görbe
 |
 |
 Ischemic heart diseases and their pathological consequences belong to the leading causes of death worldwide. In the acute treatment of myocardial ischemia one of the most important tasks is the restoration of the tissue perfusion.
Ischemic heart diseases and their pathological consequences belong to the leading causes of death worldwide. In the acute treatment of myocardial ischemia one of the most important tasks is the restoration of the tissue perfusion.
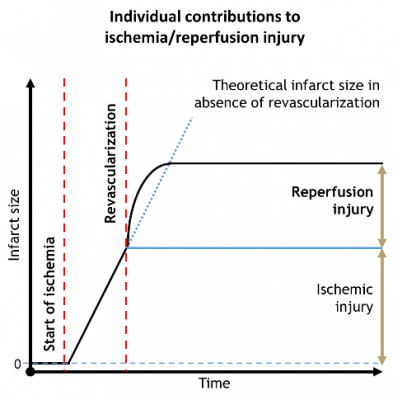
Unfortunately, reperfusion also causes further damage to the myocardium which is usually referred as reperfusion injury. In certain cases it is responsible for half of the total myocardial damage.
Despite that several small molecule drugs were found to decrease the infarct size in preclinical settings none of those were successfully translated to the human therapy. Therefore, it is necessary to find novel approaches and effective cardioprotective candidate molecules for future drug development.
Paolo Severino, Andrea D’Amato et al., 2020, Int J Mol Sci., 10.3390/ijms21218118
In the recent decades the potential application of oligonucleotide molecules such as miRNAs has emerged as an attractive and innovative approach in the treatment of various pathologies including cancers and even cardiovascular diseases.
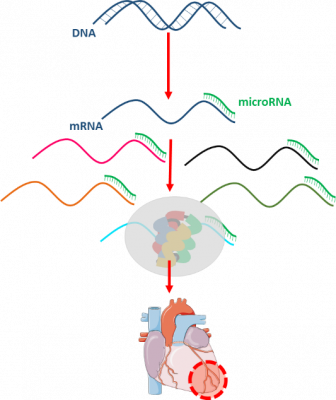 It was identified that the dysregulation of miRNAs plays an important role in the pathomechanism myocardial infarction. MicroRNAs are short, ~18-25 nucleotide-long non-coding RNA sequences those negatively regulate gene expression at the post-transcriptional level either by inhibition of the translation or promotion of the target mRNA degradation. Even entire cellular pathways can be regulated by a single microRNA, therefore miRNAs have a great potential to become multi-target drugs for diseases with multifactorial origin. Two distinct types of the microRNA therapies are microRNA mimics and antagomiRs (Varga ZV et al., Am J Physiol Heart Circ Physiol, 10.1152/ajpheart.00812.2013). MicroRNA mimics are double-stranded sequences, including “guide” strand, having the same sequence as its endogenous counterpart, and a not fully complementary passenger strand, which degrades after the detachment. AntagomiRs are essentially single-stranded antisense oligonucleotides with complementarity to an endogenous miRNA thereby blocking its function.
It was identified that the dysregulation of miRNAs plays an important role in the pathomechanism myocardial infarction. MicroRNAs are short, ~18-25 nucleotide-long non-coding RNA sequences those negatively regulate gene expression at the post-transcriptional level either by inhibition of the translation or promotion of the target mRNA degradation. Even entire cellular pathways can be regulated by a single microRNA, therefore miRNAs have a great potential to become multi-target drugs for diseases with multifactorial origin. Two distinct types of the microRNA therapies are microRNA mimics and antagomiRs (Varga ZV et al., Am J Physiol Heart Circ Physiol, 10.1152/ajpheart.00812.2013). MicroRNA mimics are double-stranded sequences, including “guide” strand, having the same sequence as its endogenous counterpart, and a not fully complementary passenger strand, which degrades after the detachment. AntagomiRs are essentially single-stranded antisense oligonucleotides with complementarity to an endogenous miRNA thereby blocking its function.
1. Explorative pharmacokinetical profiling of miRNAs in murine models
Project leader and contact person: Dr. András Makkos, Márta Szabó
 |
 |
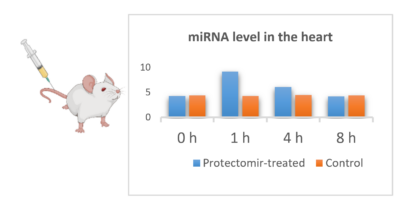 Although, the application of miRNAs are actively investigated in various indications, little is known about their pharmacokinetical properties and the differences in the characteristics between various miRNAs. Therefore, we aim to explore the pharmacokinetic profile of miRNA mimics and AntagomiRs in healthy animals.
Although, the application of miRNAs are actively investigated in various indications, little is known about their pharmacokinetical properties and the differences in the characteristics between various miRNAs. Therefore, we aim to explore the pharmacokinetic profile of miRNA mimics and AntagomiRs in healthy animals.

Learning opportunities:
- In vivo experimental methodologies (e.g., treatment with miRNAs, plasma/tissue sampling)
- In vitro methods (e.g., western blot, RNA isolation, qRT-PCR)
- Histological methods (e.g., fixation, sectioning, staining methods, RNAscope)
2. In vivo proof of concept studies of protectomiRs
Project leader and contact person: Dr. András Makkos, Márta Szabó
In these studies, we aim to explore and prove the cardioprotective effect of novel or previously identified protectomiRs (miRNAs) in murine model of myocardial infarction and heart failure. In addition, we also aim to characterize the tissue specific target gene expression profile changes both in the heart and various tissue types.
Learning opportunities:
- In vivo experimental methodologies (treatment with miRNAs, plasma/tissue sampling, murine models of myocardial infarction or heart failure)
- Histological methods (e.g. fixation, sectioning, staining methods, RNAscope)
- In vitro methodologies (e.g. western blot, RNA isolation, qRT-PCR)
3. Development of chemically modified miRNA mimics/AntagomiRs
Project leader and contact person: Dr. András Makkos, Márta Szabó
 During the development of miRNA-based pharmacotherapies it is a crucial question to provide the proper bioavailability and tissue specificity meanwhile minimizing the potential toxicity of the medication. Accordingly, we are actively working on chemical modification-based improvement of our patented cardioprotective miRNA sequences in order to improve their pharmacokinetic properties and safety.
During the development of miRNA-based pharmacotherapies it is a crucial question to provide the proper bioavailability and tissue specificity meanwhile minimizing the potential toxicity of the medication. Accordingly, we are actively working on chemical modification-based improvement of our patented cardioprotective miRNA sequences in order to improve their pharmacokinetic properties and safety.
Learning opportunities:
- In vivo experimental methodologies (treatment with miRNAs, plasma/tissue sampling)
- in silico modelling, preparative chemistry
- Cell culturing, cell culture-based experimental models (e.g. miRNA transfection, simulated-ischemia reperfusion model, viability assays, fluorescence signal measurement)
- Confocal microscopy imaging
- Histological methods (e.g. fixation, sectioning, staining methods, RNAscope)
- In vitro methodologies (e.g. western blot, RNA isolation, qRT-PCR)
4. Development of novel vehicle formulations with miRNA mimics/AntagomiRs
Project leader and contact person: Dr. András Makkos
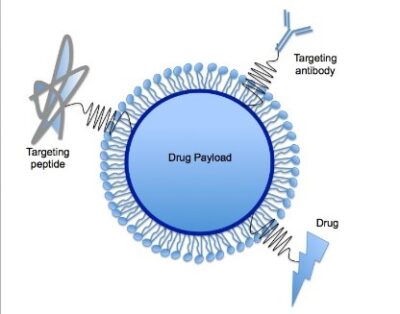 As it was mentioned earlier the proper tissue specificity and plasma/tissue half-life of the oligonucleotide-based therapies are challenging to provide. In addition to the chemical modifications the application of innovative vehicles could greatly improve the pharmacokinetic properties of cardioprotective miRNAs (e.g. tissue specifity, cellular uptake)
As it was mentioned earlier the proper tissue specificity and plasma/tissue half-life of the oligonucleotide-based therapies are challenging to provide. In addition to the chemical modifications the application of innovative vehicles could greatly improve the pharmacokinetic properties of cardioprotective miRNAs (e.g. tissue specifity, cellular uptake)
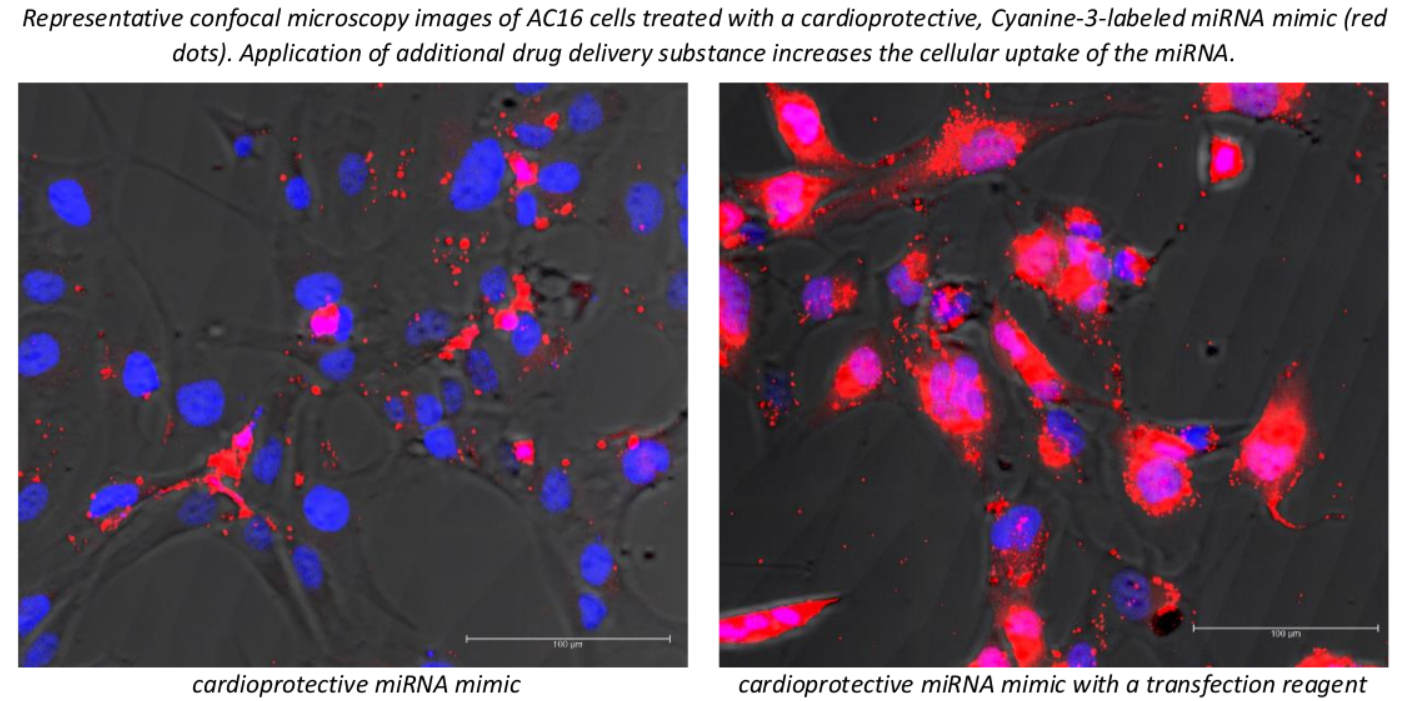
Learning opportunities:
- Cell culturing, cell culture-based experimental models (e.g. miRNA transfection, simulated-ischemia reperfusion model, viability assays, fluorescence signal measurement)
- Confocal microscopy imaging
- In vivo experimental methodologies (treatment with oligonucleotides, plasma/tissue sampling, murine models of myocardial infarction or heart failure)
- Histological methods (e.g. fixation, sectioning, staining methods, RNAscope)
Publications:
- Makkos, A., Ágg, B., Petrovich, B., Varga, Z. V., Görbe, A., & Ferdinandy, P. (2021). Systematic review and network analysis of microRNAs involved in cardioprotection against myocardial ischemia/reperfusion injury and infarction: Involvement of redox signalling. Free radical biology & medicine, 172, 237–251. https://doi.org/10.1016/j.freeradbiomed.2021.04.034
- Makkos, A., Ágg, B., Varga, Z. V., Giricz, Z., Gyöngyösi, M., Lukovic, D., Schulz, R., Barteková, M., Görbe, A., & Ferdinandy, P. (2021). Molecular Network Approach Reveals Rictor as a Central Target of Cardiac ProtectomiRs. International journal of molecular sciences, 22(17), 9539. https://doi.org/10.3390/ijms22179539
- Varga, Z. V., Zvara, A., Faragó, N., Kocsis, G. F., Pipicz, M., Gáspár, R., Bencsik, P., Görbe, A., Csonka, C., Puskás, L. G., Thum, T., Csont, T., & Ferdinandy, P. (2014). MicroRNAs associated with ischemia-reperfusion injury and cardioprotection by ischemic pre- and postconditioning: protectomiRs. American journal of physiology. Heart and circulatory physiology, 307(2), H216–H227. https://doi.org/10.1152/ajpheart.00812.2013
- Ferdinandy, P., Andreadou, I., Baxter, G. F., Bøtker, H. E., Davidson, S. M., Dobrev, D., Gersh, B. J., Heusch, G., Lecour, S., Ruiz-Meana, M., Zuurbier, C. J., Hausenloy, D. J., & Schulz, R. (2023). Interaction of Cardiovascular Nonmodifiable Risk Factors, Comorbidities and Comedications With Ischemia/Reperfusion Injury and Cardioprotection by Pharmacological Treatments and Ischemic Conditioning. Pharmacological reviews, 75(1), 159–216. https://doi.org/10.1124/pharmrev.121.000348
- Onódi, Z., Visnovitz, T., Kiss, B., Hambalkó, S., Koncz, A., Ágg, B., Váradi, B., Tóth, V. É., Nagy, R. N., Gergely, T. G., Gergő, D., Makkos, A., Pelyhe, C., Varga, N., Reé, D., Apáti, Á., Leszek, P., Kovács, T., Nagy, N., Ferdinandy, P., … Varga, Z. V. (2022). Systematic transcriptomic and phenotypic characterization of human and murine cardiac myocyte cell lines and primary cardiomyocytes reveals serious limitations and low resemblances to adult cardiac phenotype. Journal of molecular and cellular cardiology, 165, 19–30. https://doi.org/10.1016/j.yjmcc.2021.12.007
- Schreckenberg, R., Klein, J., Kutsche, H. S., Schulz, R., Gömöri, K., Bencsik, P., Benczik, B., Ágg, B., Sághy, É., Ferdinandy, P., & Schlüter, K. D. (2020). Ischaemic post-conditioning in rats: Responder and non-responder differ in transcriptome of mitochondrial proteins. Journal of cellular and molecular medicine, 24(10), 5528–5541. https://doi.org/10.1111/jcmm.15209
- Tamargo, J., Agewall, S., Borghi, C., Ceconi, C., Cerbai, E., Dan, G. A., Ferdinandy, P., Grove, E. L., Rocca, B., Sulzgruber, P., Semb, A. G., Sossalla, S., Niessner, A., Kaski, J. C., & Dobrev, D. (2023). New pharmacological agents and novel cardiovascular pharmacotherapy strategies in 2022. European heart journal. Cardiovascular pharmacotherapy, 9(4), 353–370. Advance online publication. https://doi.org/10.1093/ehjcvp/pvad034
- Weber, B. Y., Brenner, G. B., Kiss, B., Gergely, T. G., Sayour, N. V., Tian, H., Makkos, A., Görbe, A., Ferdinandy, P., & Giricz, Z. (2022). Rosiglitazone Does Not Show Major Hidden Cardiotoxicity in Models of Ischemia/Reperfusion but Abolishes Ischemic Preconditioning-Induced Antiarrhythmic Effects in Rats In Vivo. Pharmaceuticals (Basel, Switzerland), 15(9), 1055. https://doi.org/10.3390/ph15091055
- Sághy, É., Vörös, I., Ágg, B., Kiss, B., Koncsos, G., Varga, Z. V., Görbe, A., Giricz, Z., Schulz, R., & Ferdinandy, P. (2020). Cardiac miRNA Expression and their mRNA Targets in a Rat Model of Prediabetes. International journal of molecular sciences, 21(6), 2128. https://doi.org/10.3390/ijms21062128
- Vörös, I., Sághy, É., Pohóczky, K., Makkos, A., Onódi, Z., Brenner, G. B., Baranyai, T., Ágg, B., Váradi, B., Kemény, Á., Leszek, P., Görbe, A., Varga, Z. V., Giricz, Z., Schulz, R., Helyes, Z., & Ferdinandy, P. (2021). Somatostatin and Its Receptors in Myocardial Ischemia/Reperfusion Injury and Cardioprotection. Frontiers in pharmacology, 12, 663655. https://doi.org/10.3389/fphar.2021.663655
- Brenner, G. B., Makkos, A., Nagy, C. T., Onódi, Z., Sayour, N. V., Gergely, T. G., Kiss, B., Görbe, A., Sághy, É., Zádori, Z. S., Lázár, B., Baranyai, T., Varga, R. S., Husti, Z., Varró, A., Tóthfalusi, L., Schulz, R., Baczkó, I., Giricz, Z., & Ferdinandy, P. (2020). Hidden Cardiotoxicity of Rofecoxib Can be Revealed in Experimental Models of Ischemia/Reperfusion. Cells, 9(3), 551. https://doi.org/10.3390/cells9030551
- Spannbauer, A., Traxler, D., Lukovic, D., Zlabinger, K., Winkler, J., Gugerell, A., Ferdinandy, P., Hausenloy, D. J., Pavo, N., Emmert, M. Y., Hoerstrup, S. P., Jakab, A., Gyöngyösi, M., & Riesenhuber, M. (2019). Effect of Ischemic Preconditioning and Postconditioning on Exosome-Rich Fraction microRNA Levels, in Relation with Electrophysiological Parameters and Ventricular Arrhythmia in Experimental Closed-Chest Reperfused Myocardial Infarction. International journal of molecular sciences, 20(9), 2140. https://doi.org/10.3390/ijms20092140
- Sayour, N. V., Brenner, G. B., Makkos, A., Kiss, B., Kovácsházi, C., Gergely, T. G., Aukrust, S. G., Tian, H., Zenkl, V., Gömöri, K., Szabados, T., Bencsik, P., Heinen, A., Schulz, R., Baxter, G. F., Zuurbier, C. J., Vokó, Z., Ferdinandy, P., & Giricz, Z. (2023). Cardioprotective efficacy of limb remote ischaemic preconditioning in rats: discrepancy between a meta-analysis and a three-centre in vivo study. Cardiovascular research, 119(6), 1336–1351. https://doi.org/10.1093/cvr/cvad024
- Schreckenberg, R., Wolf, A., Szabados, T., Gömöri, K., Szabó, I. A., Ágoston, G., Brenner, G., Bencsik, P., Ferdinandy, P., Schulz, R., & Schlüter, K. D. (2022). Proprotein Convertase Subtilisin Kexin Type 9 (PCSK9) Deletion but Not Inhibition of Extracellular PCSK9 Reduces Infarct Sizes Ex Vivo but Not In Vivo. International journal of molecular sciences, 23(12), 6512. https://doi.org/10.3390/ijms23126512
STUDIES ON THE HIDDEN CARDIOTOXIC OR CARDIOPROTECTIVE EFFECT OF PHARMACOLOGICAL COMPOUNDS
The acute cessation of blood supply to the cardiac musculature during infarction leads to irreversible tissue damage and necrosis. These days the most efficient therapy to save the myocardium is the revascularization by either thrombolysis, percutaneous coronary intervention (PCI), or coronary bypass (CABG) surgery. However, the restoration of blood flow leads to further tissue damage. This phenomenon is termed ischemia/reperfusion injury, which can manifest in 4 ways: an increase in the amount of infarcted tissues (see Figure 1), more pronounced microvascular obstruction, increased probability of arrhythmias, decreased cardiac contractility.
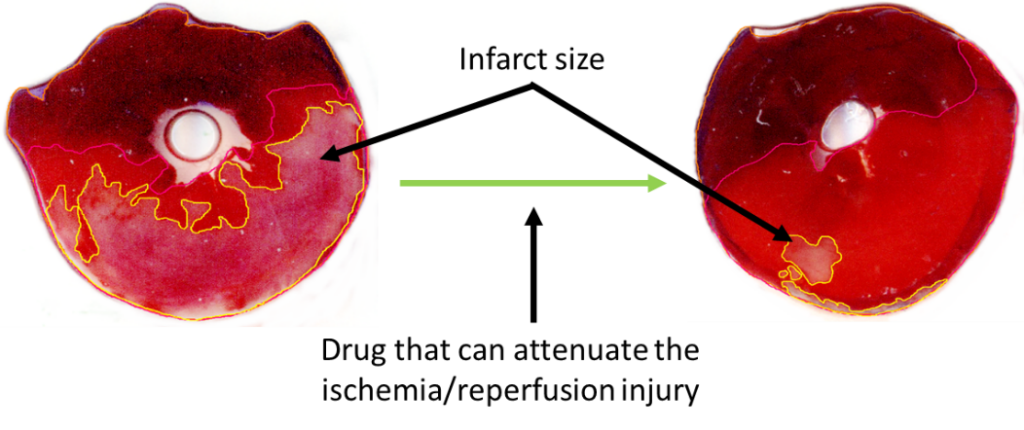
Figure 1: A slice of rat heart undergone ischemia/reperfusion and stained with Evan’s blue and TTC. Pale area on the left slice represents infarcted tissue. The area of infarcted tissue is decreased by the application of a cardioprotective pharmacological agent.
Licensed pharmacological tool for the treatment of ischemia/reperfusion injury is not yet available despite the very intensive research in the field, however, there is a significant need for them to improve acute and long-term survival and/or quality of life of patients with infarction. Therefore, we study the molecular aspects of cardiac ischemia/reperfusion injury to identify novel pharmacological targets. Furthermore, we study the interaction between pharmacons used for other symptoms and the extent of ischemia/reperfusion injury (hidden cardiotoxicity of drugs), and the effect of metabolic diseases on cardioprotective interventions and treatments.
1. Effect of pharmacological agents on ischemia/reperfusion injury in small animal models: the significance of hidden cardiotoxicity
Project supervisors: Prof. Dr. Péter Ferdinandy, Dr. Anikó Görbe Dr. Zoltán Giricz,
 |
 |
 |
Project leaders: Regina Nagy Bennet Weber Dr. Tamás Gergely
 |
 |
 |
Unexpected cardiac adverse effects are the leading causes of discontinuation of clinical trials and withdrawal of drugs from the market (see Figure 2). Since the original observations in the mid-90s, it has been well established that cardiovascular risk factors and comorbidities (such as ageing, hyperlipidaemia, and diabetes) and their medications (e.g. nitrate tolerance, adenosine triphosphate-dependent potassium inhibitor antidiabetic drugs, statins, etc.) may interfere with cardiac ischemic tolerance and endogenous cardioprotective signaling pathways. Indeed, drugs may exert unwanted effects on the diseased and treated heart that is hidden in the healthy myocardium. Hidden cardiotoxic effects may be due to (i) drug-induced enhancement of deleterious signaling due to ischemia/reperfusion injury and/or the presence of risk factors and/or (ii) inhibition of cardioprotective survival signaling pathways, both of which may lead to ischemia-related cell death and/or pro-arrhythmic effects. This led to a novel concept of ‘hidden cardiotoxicity’, defined as cardiotoxicity of a drug that manifests only in the diseased heart with e.g. ischemia/reperfusion injury and/or in the presence of its major comorbidities. Little is known on the mechanism of hidden cardiotoxicity, moreover, hidden cardiotoxicity cannot be revealed by the routinely used non-clinical cardiac safety testing methods on healthy animals or tissues. Therefore, here, we emphasize the need for development of novel cardiac safety testing platform involving combined experimental in vivo and in vitro models of cardiac diseases (especially myocardial ischemia/reperfusion and ischemic conditioning) in the presence and absence of major cardiovascular comorbidities and/or cotreatments. (Ferdinandy et al, European heart journal, 2018)
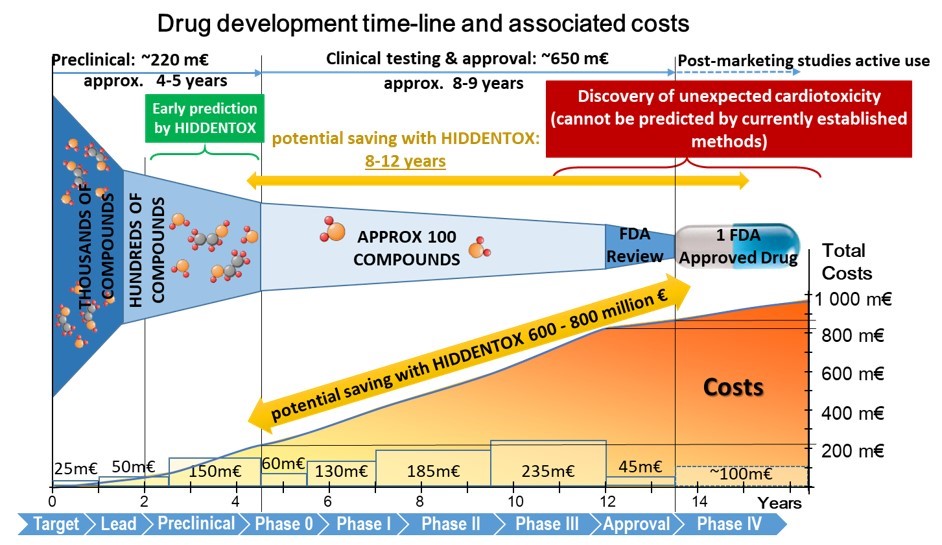
Figure 2: Cost of drug development during its phases. (Modified from: Ferdinandy et al. Eur Heart J, 2018)
Learning opportunities:
- literature search methods,
- designing in vivo rat studies,
- animal handling, oral treatment of small animals,
- performing cardiac surgeries on rats,
- performing quantitative PCR and Western blots,
- data evaluation and presentation.
Our major publications on the topic:
Ferdinandy, P. et al. Definition of hidden drug cardiotoxicity: paradigm change in cardiac safety testing and its clinical implications. Eur Heart J, 2018. (link)
Ferdinandy, P., et al. Interaction of risk factors, comorbidities, and comedications with ischemia/reperfusion injury and cardioprotection by preconditioning, postconditioning, and remote conditioning. Pharmacol Rev, 2014. 66(4): p. 1142-74. (link)
Brenner GB., et al. Hidden Cardiotoxicity of Rofecoxib Can be Revealed in Experimental Models of Ischemia/Reperfusion. Cells. 2020 Feb 26;9(3):551. (link)
Weber BY, et al. Rosiglitazone Does Not Show Major Hidden Cardiotoxicity in Models of Ischemia/Reperfusion but Abolishes Ischemic Preconditioning-Induced Antiarrhythmic Effects in Rats In Vivo. Pharmaceuticals (Basel). 2022 Aug 26;15(9):1055. (link)
Gergely TG, et al. Effects of Bempedoic Acid in Acute Myocardial Infarction in Rats: No Cardioprotection and No Hidden Cardiotoxicity. Int J Mol Sci. 2023 Jan 13;24(2):1585. (link)
2. Effect of pharmacological agents on ischemia/reperfusion injury in in vitro cell culture models
Project supervisor: Dr. Anikó Görbe
Project leaders: Bennet Weber, Regina Nagy, Márta Szabó
The hidden cardiotoxic effect of pharmacological compounds can be further tested in in vitro cell cultures. We have previously demonstrated the potential to investigate the safety of drug candidates in in vitro cell culture models in which simulation of ischemia/reperfusion (I/R) damage, and comorbidities can be achieved. Cardiac cell lines and primary isolated cardiac cells can be used for high throughput drug screening and their use is beneficial to assess the direct effects of drugs on cardiac cell types. In our project we test different agents that might show direct cardiotoxic effect on cardiac cells only in the presence of comorbidities like hypercholesterolemia or I/R injury.

Figure 3: Cell maintenance practices in the cell culture laboratory.
Learning opportunities:
- literature search methods,
- designing in vitro cell culture experiments,
- isolation of primary cardiac cells,
- maintaining cell cultures,
- treatment of cells with pharmacological compounds,
- performing viability assays,
- data evaluation and presentation.
Our major publications on the topic:
Brenner GB., et al. Hidden Cardiotoxicity of Rofecoxib Can be Revealed in Experimental Models of Ischemia/Reperfusion. Cells. 2020 Feb 26;9(3):551. (link)
Weber BY, et al. Rosiglitazone Does Not Show Major Hidden Cardiotoxicity in Models of Ischemia/Reperfusion but Abolishes Ischemic Preconditioning-Induced Antiarrhythmic Effects in Rats In Vivo. Pharmaceuticals (Basel). 2022 Aug 26;15(9):1055. (link)
3. Investigation of cardiac transcriptomic changes induced by pharmacological agents showing hidden cardiac effects
Project supervisor: Dr. Anikó Görbe
Project leaders: Bennet Weber, Regina Nagy
Endogenous cardioprotective processes and molecules are well known in the reduction of myocardial ischemia / reperfusion (I/R) damage, the effects of which are often lost during clinical translation, especially in the presence of comorbidities. In addition, many drugs may show myocardial side effects / hidden cardiotoxicity that have not been elucidated in current drug development practices. Changes in gene expression may also play a role in hidden cardiotoxicity, but these have not been fully explored and their research is a new aspect of safety pharmacology. In our project we aim to investigate the expression profile of myocardial microRNA and mRNA in rat treated with a drug with proven cardiotoxicity in an acute I/R model may result in a number of differentially expressed target molecules. Based on the revealed expression profile changes, the mechanisms responsible for the side effects can be identified. The potential toxic or protective effects of selected microRNAs can be validated in an I/R system in a human cardiomyocyte model. Molecular targets predicted in bioinformatics analysis can also be validated at transcript and protein levels.
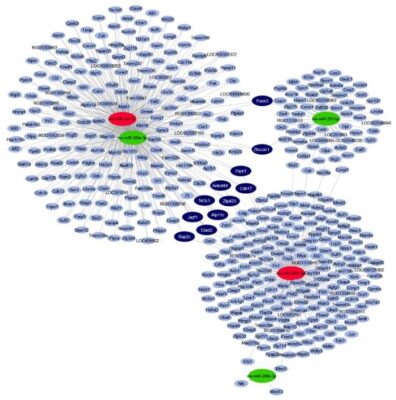
Figure 4: Interaction network and cardiac miRNA target prediction analysis of downregulated miRNAs (green) and upregulated miRNAs (red) in a rat model of prediabetes.
Learning opportunities:
- literature search methods,
- designing in vitro cell culture experiments,
- maintaining cell cultures,
- treatment of cells with pharmacological compounds,
- miRNA target search and validation by quantitative PCR and Western blots,
- data evaluation and presentation.
Our major publications on the topic:
Sághy É, et al. Cardiac miRNA Expression and their mRNA Targets in a Rat Model of Prediabetes. Int J Mol Sci. 2020 Mar 20;21(6):2128. (link)
STUDIES ON THE METABOLIC COMORBIDITIES IN CARDIOVASCULAR DISEASES
1. Effect of metabolic diseases on cardioprotective interventions and treatments against ischemia/reperfusion injury
Project supervisors: Prof. Dr. Peter Ferdinandy, Dr. Anikó Görbe
 |
 |
Project leaders: Dr. András Makkos, Dr. Tamás Gergely, Regina Nagy
 |
 |
 |
Metabolic derangements, such as obesity or diabetes, are major risk factors of cardiovascular diseases. The healthy heart can adapt to a certain level of ischemic injury (e.g., during a heart attack), but metabolic diseases, such as hypercholesterolemia, have a negative effect of this ischemia-tolerance of the heart, and may increase the extent of injury afflicted by ischemia/reperfusion. The mechanism of the changes in the myocardium due to metabolic co-morbidities are not fully understood, more detailed information on them would enable the development of novel cardioprotective therapies, which would lead to a better prognosis of ischemic heart diseases. In our Department infarction is inflicted on anesthetized high fat diet-fed rats, as a model for hypercholesterolemia, in our state of the art small animal surgery facility (Figure 1) by a surgical procedure, where the left descending coronary artery is occluded by placing a suture around it for 30-45 min then released. During surgery, we monitor vital parameters (e.g., blood pressure, ECG, temperature, respiration; Figure 2). Our undergraduate researchers learn surgical techniques and are involved actively in our ongoing studies.

Figure 1: Small animal surgery suits in the Department of Pharmacology
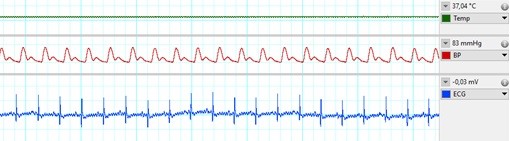
Figure 2: Vital parameters during in-vivo ischemia/reperfusion surgery. From top: body temperature, blood pressure in a central artery, ECG.
Learning opportunities:
- literature search methods,
- designing in vivo rat studies,
- working with metabolic disease models in rats,
- animal handling, oral treatment of small animals,
- performing cardiac surgeries on rats,
- performing quantitative PCR and Western blots,
- data evaluation and presentation.
Our major publications on the topic:
Andreadou I, Daiber A, Baxter GF, Brizzi MF, Di Lisa F, Kaludercic N, Lazou A, Varga ZV, Zuurbier CJ, Schulz R, Ferdinandy P. Influence of cardiometabolic comorbidities on myocardial function, infarction, and cardioprotection: Role of cardiac redox signaling. Free Radic Biol Med. 2021 Apr;166:33-52.
Andreadou I, Tsoumani M, Vilahur G, Ikonomidis I, Badimon L, Varga ZV, Ferdinandy P, Schulz R. PCSK9 in Myocardial Infarction and Cardioprotection: Importance of Lipid Metabolism and Inflammation. Front Physiol. 2020 Nov 12;11:602497. doi: 10.3389/fphys.2020.602497. PMID: 33262707; PMCID: PMC7688516.
Andreadou I, Schulz R, Badimon L, Adameová A, Kleinbongard P, Lecour S, Nikolaou PE, Falcão-Pires I, Vilahur G, Woudberg N, Heusch G, Ferdinandy P. Hyperlipidaemia and cardioprotection: Animal models for translational studies. Br J Pharmacol. 2020 Dec;177(23):5287-5311
Andreadou I, Iliodromitis EK, Lazou A, Görbe A, Giricz Z, Schulz R, Ferdinandy P. Effect of hypercholesterolemia on myocardial function, ischemia-reperfusion injury and cardioprotection by preconditioning, postconditioning and remote conditioning. Br J Pharmacol. 2017 Jun; 174 (12):1555-1569. (link)
Pavo N, Lukovic D, Zlabinger K, Zimba A, Lorant D, Goliasch G, Winkler J, Pils D, Auer K, Jan Ankersmit H, Giricz Z, Baranyai T, Sárközy M, Jakab A, Garamvölgyi R, Emmert MY, Hoerstrup SP, Hausenloy DJ, Ferdinandy P, Maurer G, Gyöngyösi M. Sequential activation of different pathway networks in ischemia-affected and non-affected myocardium, inducing intrinsic remote conditioning to prevent left ventricular remodeling. Sci Rep.2017 Mar 7;7:43958.. (link)
Nagy CT, Koncsos G, Varga ZV, Baranyai T, Tuza S, Kassai F, Ernyey AJ, Gyertyán I, Király K, Oláh A, Radovits T, Merkely B, Bukosza N, Szénási G, Hamar P, Mathé D, Szigeti K, Pelyhe C, Jelemenský M, Onódi Z, Helyes Z, Schulz R, Giricz Z, Ferdinandy. Selegiline reduces adiposity induced by high-fat, high-sucrose diet in male rats. Br J Pharmacol. 2018 Sep;175(18):3713-3726. (link)
Koncsos G, Varga ZV, Baranyai T, Boengler K, Rohrbach S, Li L, Schlüter KD, Schreckenberg R, Radovits T, Oláh A, Mátyás C, Lux Á, Al-Khrasani M, Komlódi T, Bukosza N, Máthé D, Deres L, Barteková M, Rajtík T, Adameová A, Szigeti K, Hamar P, Helyes Z, Tretter L, Pacher P, Merkely B, Giricz Z, Schulz R, Ferdinandy P. Diastolic dysfunction in prediabetic male rats: Role of mitochondrial oxidative stress. Am J Physiol Heart Circ Physiol. 2016 Oct 1;311(4):H927-H943. (link)
Baranyai, T., et al., Acute hyperglycemia abolishes cardioprotection by remote ischemic perconditioning. Cardiovasc Diabetol, 2015. 14: p. 151.

Figure 3: Cardiac ultrasonography and cardiac pressure-volume analysis in the small animal surgery suits.
2. Effect of hypercholesterolemia on inflammasome activation in macrophages
Project supervisors: Dr. Anikó Görbe, Dr. Zoltán Varga
Project leader: Regina Nagy
Hypercholesterolemia induces cholesterol accumulation in immune cells, like macrophages, which promotes inflammation through Toll-like receptor (TLR) signaling and inflammasome activation. Intracellular cholesterol accumulation and the consequent inflammatory response is probably beneficial in the response to infection, however, it worsens diseases in association with chronic inflammation, like atherosclerosis. Inflammation also plays a critical role in the genesis, progression, and manifestation of several cardiovascular diseases. Direct inhibition of inflammasomes, rather than proinflammatory cytokine suppression, can be an effective anti-inflammatory management. Thus, exploring the molecular mechanisms behind inflammasome activation can lead to the discovery of new potential drug targets in hypercholesterolemic cardiovascular comorbidities. In our project we aim to investigate the effect of in vitro hypercholesterolemic treatment on the inflammatory mechanisms of human monocytes/macrophages.
Learning opportunities:
- literature search methods,
- working with metabolic disease models in cell cultures,
- designing in vitro cell culture experiments,
- maintaining cell cultures,
- treatment of cells with pharmacological compounds,
- performing quantitative PCR and Western blots,
- data evaluation and presentation.
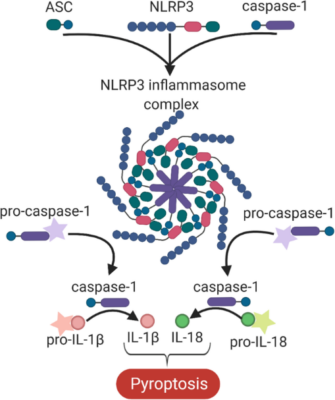
Figure 4: The NLRP3 inflammasome complex.
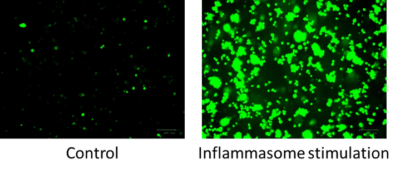
Figure 5: Fluorescent images of THP1-ASC-GFP monocytes following inflammasome stimulation by flagellin.
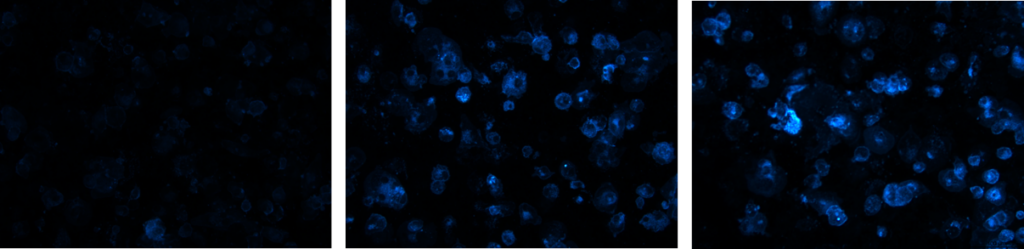
Figure 6: Fluorescent images of cholesterol uptake of THP1-ASC-GFP cells after treatment with different concentrations of hypercholesterolemic supplement.
Members
Group Leader:
Anikó Görbe, MD, PhD
Researchers/Postdocs:
Orsolya Somogyi, MSc
András Makkos, MD, PhD
Éva Sághy, MD, PhD
PhD Students:
Regina Nagy, MSc
Márta Szabó, MSc
Bennet Weber, MD
Assistants:
Tünde Petrovics, MSc
László Horváth, MSc, PhD
Orsolya Szalai, MSc
Reyad Ali








