Projects
Study of inflammatory and immunological processes in cardiovascular diseases
The prognosis for chronic heart failure, even with modern therapeutic tools and drugs, is quite poor; about half of the patients die within 5 years of the diagnosis. Treatment of the disease puts a heavy burden on health systems. A variety of factors can lead to heart failure, but during its development, the heart muscle’s structure and functionality suffer damage. The most common causes include ischemic heart disease, genetic and drug-induced (most often anti-tumor chemotherapies) cardiomyopathies, but arrhythmias and valve disorders can also cause its development. Despite the different etiology, important roles in the progression of heart failure play both cellular (neutrophilic granulocytes, macrophages, B and T lymphocytes) and humoral inflammatory processes, inflammation-inducing cytokines, neuropeptides such as IL-1β, IL-18, or TNF-α, which significantly increase damage to the heart muscle.

The point of our research is to look into how these types of cells and different humoral factors can be used to treat heart failure in different animal models and in vitro cell-based testing systems.
- Study of inflammatory processes in the development of heart muscle dysfunction associated with metabolic diseases (obesity, diabetes, hypercholesterolemia, liver cirrhosis) and aging
Project Leaders: Dr., Viktória Tóth, Dr., Zoltán V. Varga
Heart failure is one of the leading causes of death worldwide. Clinically, the disease can occur in two distinct forms: with reduced ejection fraction (HFrEF) or preserved ejection fraction (HFpEF). Nearly half of patients with heart failure have HFpEF, i.e., the ejection fraction is retained. These patients are usually elderly and often suffer from several concomitant conditions (e.g., diabetes, obesity, hypercholesterolemia). In many cases, patients do not respond well to medicines used in the treatment of HFrEF (digitalis, ACE inhibitors, β-blockers), which is why the development of medicines in this area is very significant. In addition, HFpEF is characterized by high mortality (5-year mortality is 65%).
The molecular mechanism for the formation of HFpEF is little known, but a better understanding of the outcome of the disease is essential. It is assumed that a systemic inflammatory condition may contribute to cardiovascular dysfunction, but its causal role has not yet been established. Our current knowledge is further limited by the difficulty of animal-based modeling of this complex disease.The molecular mechanism for HFpEF formation is little known, but a better understanding of the disease’s outcome is essential. Although we assume a systemic inflammatory condition may contribute to cardiovascular dysfunction, we have not yet established its causal role. The difficulty of animal-based modeling of this complex disease further limits our current knowledge.
Our goal is to develop and test new animal models that allow translational testing of HFpEF.
Learnable Techniques:
- Working with experimental animals.
- histological techniques, immunohistochemistry,
- RNA and protein work – Western blot, qRT-PCR
References:
- Valenta I*, Varga ZV*, Valentine H, Cinar R, Horti A, Mathews WB, Dannals RF, Steele K, Kunos G, Wahl RL, Pomper MG, Wong DF, Pacher P, Schindler TH. Feasibility Evaluation of Myocardial Cannabinoid Type 1 Receptor Imaging in Obesity: A Translational Approach. JACC Cardiovasc Imaging. 2018 Feb;11(2 Pt 2):320-332. [IF: 10.189] *-megosztott elsőszerzőség
- Varga ZV, Kupai K, Szűcs G, Gáspár R, Pálóczi J, Faragó N, Zvara A, Puskás LG, Rázga Z, Tiszlavicz L, Bencsik P, Görbe A, Csonka C, Ferdinandy P, Csont T.: MicroRNA-25-dependent up-regulation of NADPH oxidase 4 (NOX4) mediates hypercholesterolemia-induced oxidative/nitrative stress and subsequent dysfunction in the heart. J Mol Cell Cardiol. 2013. 62:111-21. [IF: 5.218]
- Cardio-Oncology and Onco-Cardiology
Project Leaders: Dr., Tamás Gergely, Dr., Nabil Sayour, Tamás Kovács, Dr., Zoltán V. Varga
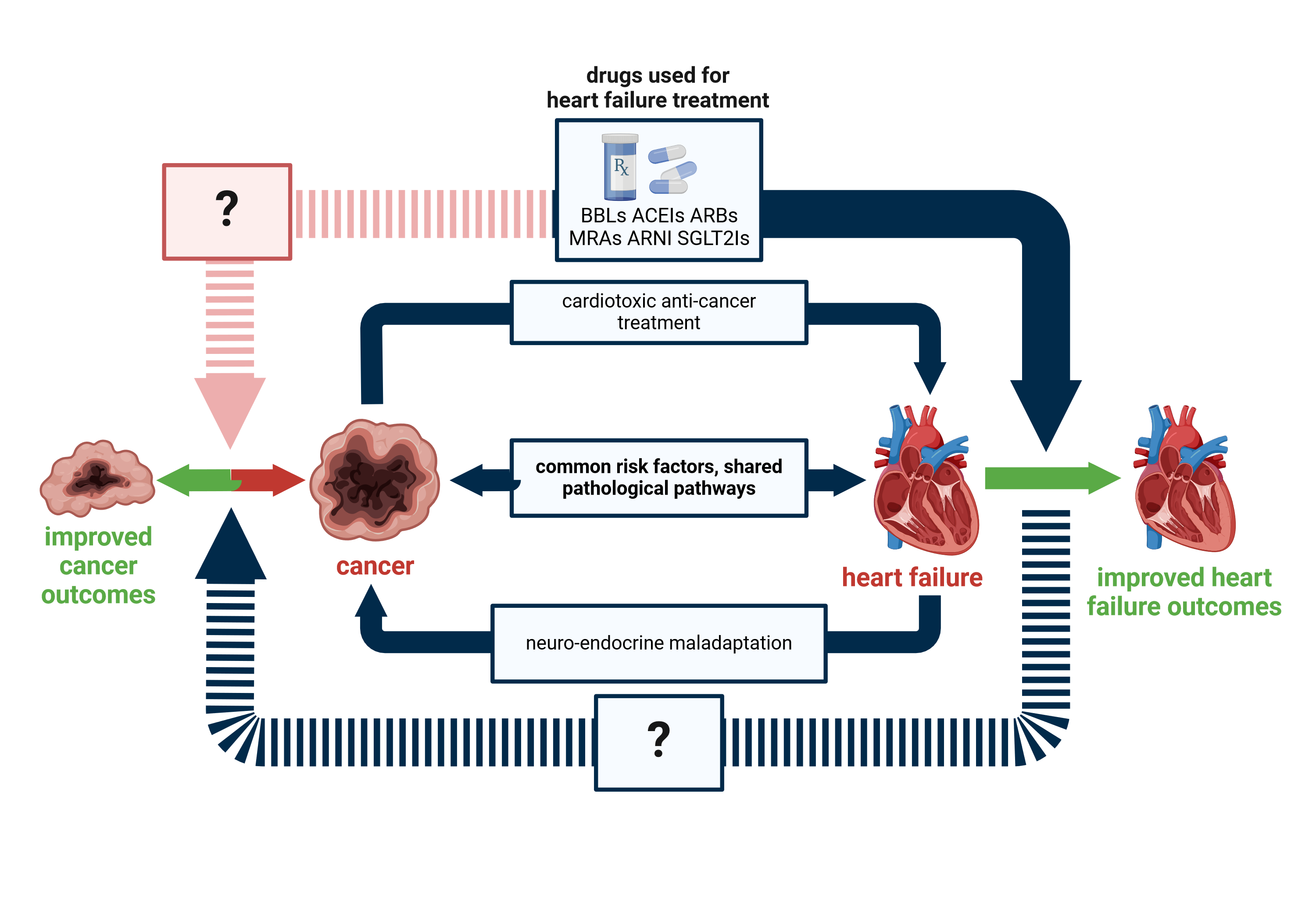
Cardiovascular and tumor diseases frequently coexist and develop in the same patient. These two groups of diseases account for half of all deaths worldwide, so it is not surprising that attention is paid to the simultaneous occurrence of the two serious conditions. Cardiovascular and oncological diseases have common risk factors, they can develop through similar pathomechanisms, but in some cases they can also have a common genetic background, not to mention the possible side effects of the treatments used. This is the subject of cardiology-oncology.
In recent decades, significant advances have been made in anti-cancer therapies. The introduction of immunotherapies significantly improved the survival of ongological patients. Immune Checkpoint Inhibitors (ICIs) are the most common type of immunotherapy. These monoclonal antibodies reverse the inhibition of immune cell activation by the tumor and thus contribute to the destruction of cancer cells through an increased adaptive immune response. It is showing the importance of the field that for the discovery of CTLA-4 and PD-1 receptors, James P. Allison and Tasuko Honjo won the 2018 Nobel Prize in Medicine.

The activation of the immune system in the above-mentioned manner is non-specific, and ICI therapy can also trigger the overactivation of immune cells in non-tumor tissues, thereby leading to immune-related adverse events (irAEs). The rarest, but also the most severe side effects are myocarditis and heart failure.
Our main objective is to understand the processes behind heart muscle damage, which appears as a rare side effect. In this way, new diagnostic and therapeutic targets can be identified and influenced to reduce the incidence and severity of the adverse effects of immune checkpoint inhibitors. Particular emphasis is placed on the study of the role of inflammatory cells activated in the thymus, their role in the development of toxicity (1). Our objectives include, in addition to the treatment of the cardiotoxic side effects of immune checkpoint inhibitors, the development of a combination treatment that does not affect the tumor response and can even enhance the anti-tumor effect.
Ongoing projects:
- Study of the interaction between tumor disease and heart failure
- Antitumor therapy-induced heart failure investigation and treatment possibilities
Heart failure itself is a hard-to-influence, prolonged disease that can develop for various reasons. Many medicines are used to treat it, however, the life prospects of patients are poor, so additional new and effective drugs are needed. Our research team uses animal testing to model heart failure and find new drug targets.
Ongoing projects:
- Drug-induced heart dysfunction model and examination of pharmacological treatment possibilities
- Study of therapeutic possibilities for heart failure in the pressure overload heart failure model
- Effects of aging on drug-induced heart failure
Certain heart diseases (e.g. heart failure mentioned above) are already an oncogenic environment in themselves, which increases the risk of developing the tumor. Such a reverse approach, where the effect of factors from the heart on the tumor is examined, is called reverse onco-cardiology or cardio-oncology. Our research team is conducting studies to find out whether pharmacotherapy for heart failure or heart failure increases the risk of tumor development, and how the growth of emerging tumors changes in the case of existing heart failures, and what molecular processes may be behind them. We aim to formulate new treatment recommendations for these patients.
Ongoing projects:
- Influence of heart failure on tumor growth
- Effect of pharmacotherapy for heart failure on tumor growth
Learnable Techniques:
- in vitro cell culture and experimental systems
- Spectral flow cytometry, immunophenotyping
- Working with experimental animals
- Creation and testing of tumor models in vivo
- monitoring of tumor growth using imaging techniques (tumor scanner, ultrasound, in vivo fluorescence and bioluminescence studies)
- histological techniques, immunohistochemistry, immunofluorescence
Our publications related to the topic:
- Gergely TG, Kucsera D, Tóth VE, et al. Characterization of immune checkpoint inhibitor-induced cardiotoxicity reveals interleukin-17A as a driver of cardiac dysfunction after anti-PD-1 treatment. Br J Pharmacol. 2023;180(6):740-761. doi:10.1111/bph.15984
- Gergely TG, Drobni ZD, Sayour NV, Ferdinandy P, Varga ZV. Molecular fingerprints of cardiovascular toxicities of immune checkpoint inhibitors. Basic Res Cardiol. Published online July 17, 2024. doi:10.1007/s00395-024-01068-8
- Gergely TG, Drobni ZD, Kallikourdis M, et al. Immune checkpoints in cardiac physiology and pathology: therapeutic targets for heart failure. Nat Rev Cardiol. 2024;21(7):443-462. doi:10.1038/s41569-023-00986-9
Members
Group leader:
Researchers/Postdocs:
Tóth Viktória, PharmD, PhD
Szabó Lilla, MSc
Kovács Tamás, MSc
Anna Gelencsérné Kulin, MSc
Zoltán Márton Köhler, MD, PhD
PhD students:
Gergely Tamás, MD
Viktor Nabil Sayour, MD
Jakab Márk, MD
Kocsis Márton, MSc
Hegedűs Zsombor, MSc
Ayham Alhaddad, MD
Assistant:
Szabolcs Farkas
Kovács Andrea, MSc
Gallery





