Projects
Investigation of inflammatory and immunological processes in cardiovascular diseases
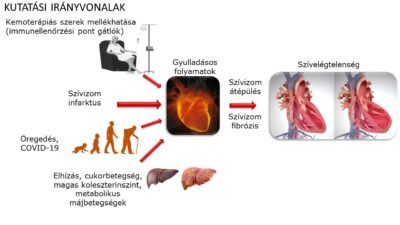
Even with modern therapeutic tools and drugs, the prognosis for chronic heart failure is still very poor, with around half of patients dying within 5 years of diagnosis. Heart failure can be caused by a number of factors, but its onset is associated with damage to the structure and functionality of the heart muscle. The most common causes include ischaemic heart disease, genetic and drug-induced cardiomyopathies, but arrhythmias and valve disease can also cause it. Despite the different aetiology, both cellular (neutrophil granulocytes, macrophages, B and T lymphocytes) and humoral inflammatory processes, pro-inflammatory cytokines, neuropeptides such as IL-1β, IL-18 or TNF-α play an important role in the progression of heart failure and significantly enhance myocardial damage. Our studies aim to investigate the therapeutic modulation of these cell types and different humoral factors in different heart failure models, and in vitro cell-based screening platforms.
1.1 The role of different inflammatory mechanisms (inflammasome priming and activation, DNA sensor pathways) and their therapeutic potential in cardiac diseases
Project leaders: Zsófia Onódi, MD, PhD, Zoltán Varga, MD, PhD
Background: IL-1β release is regulated by inflammasomes. Inflammasomes are multiprotein complexes expressed by different immune cells that induce the cleavage and release of cytokines such as IL-1β and IL-18. When pathogen- or danger-associated patterns appear, immune cells express inflammasome proteins in increased amounts to prepare for an immune response (a phenomenon known as inflammasome priming). When intracellular inflammasome sensors (such as NLRP3, NLRC4 or AIM2) recognise specific patterns, inflammasomes assemble and activate the enzyme caspase-1, leading to the cleavage of cytokines (this is called inflammasome “activation” or “triggering“). Our previous studies have shown that inflammasomes may play an important role in the pathomechanism of HF, as we found increased protein expression of inflammasome sensors AIM2 and NLRC4 in human heart samples from HF patients, which was confirmed by the detection of IL-1β [1, 2]. Furthermore, we have shown that probenecid, a clinically used anti-gout drug, has significant anti-inflammatory, in particular inflammasome inhibitory effects in in vitro and in vivo models [1]. According to literature data [3], probenecid may act by modulating purinergic signalling through inhibition of the pannexin-1 channel, which may also inhibit inflammasome priming and activation. However, it is still not known which step(s) probenecid and other inflammasome inhibitors may inhibit specifically.
Objectives:
- To characterize changes in inflammatory pathways in the heart and other tissues using myocardial dysfunction and heart failure animal models.
- To establish a complex in vitro drug screening platform to test inflammasome inhibitors,
- Test drugs already approved by regulatory authorities on the established in vitro platform for further in vivo studies.
1.2 Morphological and functional changes of immune organs (bone marrow, spleen, thymus) in heart disease – mechanisms and therapeutic options
Project leaders: Zsófia Onódi, MD, PhD, Zoltán Varga, MD, PhD
Background: The main sites of inflammatory and immune cell homeostasis are the haematopoietic and immune organs such as bone marrow, spleen, thymus and lymph nodes. Several studies are available on the increased inflammatory activity in the heart or plasma of HF patients and animals, but the alterations and consequences of inflammatory pathways in extracardiac organs are less well understood [4]. It has been known for many years that cardiac diseases, such as advanced HF, are associated with haematopoietic dysfunction, including anaemia or altered leukocyte counts, which are associated with a worse prognosis [5, 6]. Recently, it has been reported that heart disease can induce significant changes in bone marrow that may affect blood and immune cell production, leading to increased inflammation [7]. Despite a growing body of literature on immune and haematopoietic dysfunction associated with HF, there is a lack of data on the effects of the pro-inflammatory environment in heart disease. Therefore, a detailed characterization of the inflammatory activity of hematopoietic organs is needed in the future.
Objectives:
- to study inflammatory processes in the heart and immune organs such as bone marrow, thymus and spleen,
- to assess changes in the activity of inflammatory pathways in immune organs from different aetiologies (e.g. transverse aortic stenosis or drug-induced) in HF mouse and rat models.
Our experimental approach:
- Animal models: rats or mice are used for the experiment. We induce myocardial damage and thus myocardial dysfunction by various methods, e.g. infarction, pressure-overload heart failure by aortic constriction or myocardial damage by cardiotoxic drugs. Animals are followed up for a limited period of time, blood samples are collected and cardiac function is assessed by echocardiography, and finally tissue samples are collected.
- Cell culture models: to study the potential inflammasome inhibitory effect of drugs, the following experiments are performed in vitro. THP1 native or ASC-GFP reporter cell lines are used to stimulate inflammasome protein expression (priming) and induce inflammasome activation. After treatment, cells and supernatants are harvested, homogenised or prepared for imaging.
- Histology, immunohistochemistry and immunofluorescence: Classical histological staining is used to assess the presence of organ fibrosis and fatty deposits. Cell surface markers and intracellular protein expression are assessed and colocalisation studies are performed on tissue sections. Classical microscopy techniques or confocal microscopy are used for imaging.
- In vitro molecular and biochemical measurements: cell signalling changes, protein and cytokine levels, enzyme activities are determined by Western blot, qRT-PCR, ELISA, cytokine array or enzyme activity assays.
References:
- Onodi, Z., et al., AIM2-driven inflammasome activation in heart failure. Cardiovasc Res, 2021. 117(13): p. 2639-2651.
- Kugler, S., et al., Inflammasome activation in end-stage heart failure-associated atrial fibrillation. ESC Heart Fail, 2022. 9(4): p. 2747-2752.
- Onodi, Z., et al., Drug repurposing for cardiovascular diseases: New targets and indications for probenecid. Br J Pharmacol, 2023. 180(6): p. 685-700.
- Li, H., C. Chen, and D.W. Wang, Inflammatory Cytokines, Immune Cells, and Organ Interactions in Heart Failure. Front Physiol, 2021. 12: p. 695047.
- Anand, I.S. and P. Gupta, Anemia and Iron Deficiency in Heart Failure: Current Concepts and Emerging Therapies. Circulation, 2018. 138(1): p. 80-98.
- Sukhbaatar, N. and T. Weichhart, Iron Regulation: Macrophages in Control. Pharmaceuticals (Basel), 2018. 11(4).
- Rohde, D., et al., Bone marrow endothelial dysfunction promotes myeloid cell expansion in cardiovascular disease. Nat Cardiovasc Res, 2022. 1(1): p. 28-44.
Fig1 – Adamo, L., Rocha-Resende, C., Prabhu, S.D. et al. Reappraising the role of inflammation in heart failure. Nat Rev Cardiol 17, 269–285 (2020). https://doi.org/10.1038/s41569-019-0315-x
Fig2 – Solimando AG, Melaccio A, Vacca A, Ria R. The bone marrow niche landscape: a journey through aging, extrinsic and intrinsic stressors in the haemopoietic milieu. Journal of Cancer Metastasis and Treatment. 2022; 8: 9. http://dx.doi.org/10.20517/2394-4722.2021.166