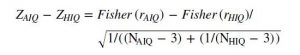NeuroImage 146 (2017) 554-560
DOI: 10.1016/j.neuroimage.2016.09.039
Adrián Pótári a,1, Péter P. Ujma b,i,1, Boris N. Konrad c, Lisa Genzel d, Péter Simor a,e, János Körmendi f, Ferenc Gombos g, Axel Steiger h, Martin Dresler c,h,1, Róbert Bódizs b,1
a Department of Cognitive Sciences, Budapest University of Technology and Economics, H-1111 Budapest, Hungary
b Institute of Behavioural Sciences, Semmelweis University, H-1089 Budapest, Hungary
c Donders Institute for Brain, Cognition and Behaviour, Radboud University Medical Centre, 6525 EN Nijmegen, The Netherlands
d Centre for Cognitive and Neural Systems, University of Edinburgh, EH8 9JZ Edinburgh, United Kingdom
e Nyírő Gyula Hospital, National Institute of Psychiatry and Addictions, H-1135 Budapest, Hungary
f Department of Electrical Engineering and Information Systems, Pannon University, H-8200 Veszprém, Hungary
g Department of General Psychology, Pázmány Péter Catholic University, H-1088 Budapest, Hungary
h Max Planck Institute of Psychiatry, 80804 Munich, Germany
i National Institute of Clinical Neurosciences, H-1145 Budapest, Hungary
Abstract
Impaired sleep is a frequent complaint in ageing and a risk factor for many diseases. Non-rapid eye movement (NREM) sleep EEG delta power reflects neural plasticity and, in line with age-related cognitive decline, decreases with age. Individuals with higher general intelligence are less affected by age-related cognitive decline or other disorders and have longer lifespans. We investigated the correlation between age and EEG power in 159 healthy human subjects (age range: 17–69 years), and compared an average (IQ<120; N=87) with a high (IQ≥120; N=72) intelligence subgroup. We found less age-related decrease in all-night relative NREM sleep EEG delta power in the high intelligence subgroup. Our results suggest that highly intelligent individuals are less affected by the sleep-related effects of biological ageing, and therefore potentially less at risk for age-related cognitive deficits and other diseases.
Keywords: sleep, electroencephalography, slow wave activity, aging, intelligence, fluid reasoning
1.Introduction
Sleep is an indispensable physiological state that has an essential role in the maintenance and restoration of neural functions underlying cognitive and affective processes. Ageing is related to increasing sleep disturbances, which in turn show co-morbidities with many psychiatric and neurological diseases – not only as a symptom, but in many cases as a risk factor (Dresler et al., 2014).
Sleep EEG slow waves ( < 4.5 Hz) are an index of sleep pressure and a hallmark of NREM sleep function (Achermann et al., 1993; Tononi and Cirelli, 2014). Slow wave activity dissipates during successive sleep cycles, increases in recovery sleep after sleep deprivation (Borbely et al., 1981) and exhibits local changes related to experience-dependent plasticity (Kattler et al., 1994; Huber et al., 2006, 2004) during preceding wakefulness, all evidence for homeostatic regulation. There is strong evidence that slow wave activity reflects changes in synaptic strength in the cortex and can thus be considered an index of synaptic plasticity (Tononi and Cirelli, 2014).
In line with the hypothesis that slow waves reflect cortical homeostatic processes, slow wave activity was shown to correlate with cortical thickness both during adolescence (Buchmann et al., 2011) and adulthood (Mander et al., 2013). Within both age ranges, ageing is characterized by both cortical thinning (Tamnes et al., 2010; Raz, 2000) and a decrease in slow wave activity (Carrier et al., 2001; Feinberg and Campbell, 2013; Kurth et al., 2010a 2010b). Retained slow wave sleep at a high age is associated with better neurocognitive functioning (Anderson and Horne, 2003) as well as lower mortality (Mazzotti et al., 2014). While probably due to different mechanisms, shorter (Geiger et al., 2010) or optimal length(Kocevska et al., 2016) sleep and longer N2 sleep duration (Busby and Pivik, 1983) in children was also found to be associated with higher IQ, highlighting the importance of sleep among the biological correlates of intelligence.
High intelligence is associated with better outcomes concerning the adverse effects of ageing. Higher educational status and intelligence are protective factors against dementia in old age, with intelligence probably having the greater predictive value (Meng and D’Arcy, 2012; Schmand et al., 1997). High intelligence is associated with attenuated age-related thinning of the frontal cortex and a more pronounced cortical thickening over time, with genes influencing variability in both intelligence and brain plasticity partly driving these associations (Brans et al., 2010). In addition, highly intelligent individuals have greater longevity (Hauser and Palloni, 2011; Batty et al., 2006) and a lower occurrence of heart disease (Batty et al., 2008)and cancer (Batty et al., 2009).
The association between ageing, cognition, and sleep, however, still remains poorly understood (Yaffe et al., 2014). Based on protective effects of intelligence against ageing for other measures, we hypothesized that sleep – especially NREM slow wave activity – is less affected by ageing in highly intelligent individuals. To test this hypothesis, we compared full-night sleep EEG recordings of an average intelligence sample (AIQ: IQ < 120; N=87) with a high intelligence sample (HIQ: IQ≥120; N=72) across the lifespan (age range: 17–69 years).
2.Materials and methods
2.1. Participants
Across sites (Max Planck Institute of Psychiatry, Munich, Germany; Institute of Behavioural Sciences of Semmelweis University, Budapest, Hungary) of this retrospective multicenter study, polysomnography data from 168 subjects was collected. Nine subjects were excluded from the analysis due to the excessive presence of artifacts in their recordings. Therefore, the study was performed using a sample of 159 subjects (72 females, 87 males, mean age=29.46 years, age range: 17–69 years). The research protocols were approved by the Ethical Committee of the Semmelweis University, Budapest, or the Ludwig Maximilian University, Munich, in accordance with the Declaration of Helsinki. All subjects signed informed consent for the participation in the studies. According to a semi-structured interview with experienced psychiatrists or psychologists, all subjects were healthy, had no history of neurological or psychiatric disease, and were free of any current drug effects excluding contraceptives. Consumption of small habitual doses of caffeine (max. 2 cups of coffee before noon), but no alcohol was allowed before the recordings. 6 male and 2 female subjects were light to moderate smokers (self-reported), while the rest of the subjects were non-smokers.
2.2. Sleep recordings and preprocessing
Sleep was recorded on the second night (after one adaptation night) by standard polysomnography. Common recording sites across studies and laboratories were: Fp1, Fp2, F3, F4, Fz, F7, F8, C3, C4, Cz, P3, P4, T3, T4, T5, T6, O1, and O2, electro-oculography (EOG), bipolar submental electromyography (EMG), as well as electrocardiography (ECG). EEG electrodes were re-referenced to the mathematicallylinked mastoids. Impedances for the EEG electrodes were kept below 8 kΩ. Sampling frequency was either 249 Hz, 250 Hz or 1024 Hz, depending on recording site. All recorded data were corrected for different filter characteristics across sites (see our previous article (Ujma et al., 2014) for details, also on sampling frequency) by applying a fixed-amplitude signal from an analogue signal generator, measuring the amplitude of the devices’ recorded digital signals and calculating their amplitudes at 0.05 Hz, every 0.1 Hz between 0.1 and 2 Hz, every 1 Hz between 2 and 20 Hz, every 10 Hz between 10 and 100 Hz, resulting in the device’s individual hardware filter characteristics profile. We determined the amplitude reduction rate of each recording system by calculating the proportion between digital (measured) and analog (generated) amplitudes of sinusoid waves for both inducing (40 and 355 μV amplitude) signals. Machine-specific amplitude reduction rates were given as the mean amplitude rate between digital and analog values at the two (40 and 355 μV) amplitudes (frequency response). Frequency-specific reduction was compensated by multiplying power in each frequency bin by a factor corresponding to the squared inverse of the frequency response of the amplifier.
Sleep EEG recordings were manually scored by an experienced rater on a 20 s basis by applying standard criteria (Iber et al., 2007). A 20 second epoch length was chosen to reduce impreciseness due to mid-epoch changes in sleep stage and to ensure an overlap with the 4 s artifact epochs (see later in this section). Sleep cycles (NREM-REM cycles) were defined by using validated criteria (Aeschbach and Borbely, 1993). A minimum of 15 min of NREM sleep followed by minimum 5 min of REM sleep was considered a sleep cycle. Exception for the criteria of the minimum 5 min of REM duration was allowed in the case of the first sleep cycle (containing short REM periods in many young subjects). Artifacts were removed on a 4 s basis by visual inspection of all recorded channels. In case of electrode failures data from the affected electrodes were not analyzed. If excessive artifacts were continuously seen on an electrode, artifact rejection was not performed on this electrode, but instead all data was excluded. For 20 subjects (Munich-II setup), some electrodes (Fz, Cz, F7, F8, T3, T4, T5, T6) were not available by default and they were treated as missing data. The number of available subjects for each electrode is indicated in Table 2. In case of ECG artifacts (N=5) EEG data was used after removing these artifacts using independent component analysis implemented in EEGLAB (Swartz Center for Computational Neuroscience, San Diego, CA), an add-on to MATLAB (MathWorks, Natick, MA).
2.3. Spectral analysis and spectral bands
Artifact-free epochs of sleep stages N2 and slow wave sleep (SWS) were analyzed to obtain NREM spectral data, and artifact-free REM epochs were analyzed to obtain REM spectral data. Spectral analysis was performed by the mixed-radix FFT method using 4 s Hanningtapered windows with a 2 s overlap and averaging power spectral densities from all-night 4 s windows for NREM and REM phases separately. Power spectral densities were calculated for 0.25 Hz bins from 0 Hz to the Nyquist frequency (sampling rate/2). In addition, sleep cycle-specific power spectral analyses on NREM sleep episodes of the first four cycles were performed. We computed EEG band power spectral power density for the delta (0.5–4 Hz), theta (4–7.5 Hz), alpha (7.5–11 Hz), sigma (11–15 Hz) and beta (15–30 Hz) bands by Simpson’s rule of numerical integration. Data below 0.5 Hz was not included in analyses due to frequent contamination with perspiration artifact. Relative power for these bands was computed by dividing band power with total power (the sum of band power values in the analyzed 0.5–30 Hz range). Since relative power in the higher bands were expected to be heavily influenced by power in the delta band, which has the highest spectral power, only the delta band was considered for relative power analyses.
2.4. Intelligence testing
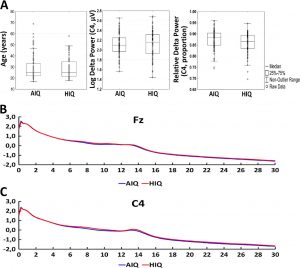
Based on their availability to participate, subjects completed one, two or three standardized nonverbal intelligence tests. The tests used in the study were the Culture Fair Test (CFT; (Cattell, 1973)), Raven Advanced Progressive Matrices (Raven APM; (Raven et al., 2004)) and the Number Connection Test (Zahlenverbindungstest, ZVT; (Oswald and Roth, 1987)). ZVT was used because of its available IQ norms (Oswald and Roth, 1987) and high correlation with other IQ tests (Mackintosh, 2011; Williams and Pearlberg, 2006) (in our sample rZVTCFT=−0.663 and rZVT-Raven=−0.574, p < 0.001 each). Both the CFT and Raven APM are nonverbal intelligence tests in which subjects are required to complete abstract patterns by finding their organizing rules, while the ZVT is a classical trail making task where numbers have to be connected as quickly as possible. Performance in these tests was shown to correlate strongly and to be a particularly good measurement of the general factor of intelligence (Cattell, 1973; Duncan et al., 2000; Prokosch et al., 2005). A total of 111 subjects completed the CFT, 81 subjects completed the Raven APM test and 101 subjects completed the ZVT. All tests yielded compatible IQ scores, which were averaged in case of multiple available tests for a single subject. Based on their mean IQ score, the sample was split into an average (AIQ: IQ < 120; N=87) and a high intelligence (HIQ: IQ≥120; N=72) subgroup. The cutoff point of IQ=120 was chosen for three reasons: 1) this was in line with previous studies about the EEG correlates of intelligence (Thatcher et al., 2007; Thatcher et al., 2005) 2) it was possible to form two homogeneous (in terms of age and sex), sufficiently large subsample of roughly similar sizes on the two sides of this cutoff point 3) an IQ of 120 corresponds to approximately the 75% percent of the distribution of IQ scores of people working in professions which require college degrees (Hauser, 2002), realistically suggesting that this IQ level is representative of people working in the most intellectually demanding jobs and performing above the average, a practical definition of ‘highly intelligent individuals’. There was no difference between the AIQ (N=87) and HIQ (N=72) groups in terms of age (MeanAIQ=29.9 years; MeanHIQ=29.0 years, t=0.54, p=0.59). In the AIQ group, 36 of subjects (41%) were females, while in the HIQ group, 36 subjects (50%) were females. This difference was not statistically significant. Detailed descriptive statistics of age and IQ level (as well as EEG spectral power) are available in Table 1 and represented in Fig. 1. Fig. 1 also shows the mean EEG spectrum of each subgroup.
Table 1
Detailed descriptive statistics of absolute (log 10 μV2) and relative spectral power (proportion) on C4, age and IQ (upper section). Distribution of IQ scores among participants (lower section).
Fig. 1. Panel A: detailed descriptive statistics of age, absolute log power on C4 and relative log power on C4. Boxplots indicate the medians and distribution quartiles, while whiskers indicate the outlier range. Markers indicate data points. Additionally, mean values of absolute power (log 10 μV2) on Fz (panel B) and C4 (panel C) are shown by subgroup.
2.5. Statistics
We computed Spearman’s rank correlation coefficients between age, sleep macrostructure variables and relative as well as logarithmized (10-base) absolute EEG band power spectral density independently for the average and high IQ groups. We calculated the differences between correlation coefficients using the following formulas (with r indicating correlation coefficients and N indicating sample size).
First, we used Fisher’s r-to-z method to normalize the distribution of r values:
then calculated the z-scores for the Fisher z-transformed correlation coefficients:
and calculated the z-score of the difference of the normalized correlation coefficients:
Multiple comparison correction of these comparative statistics was performed using the Benjamini-Hochberg method for false discovery rate correction (Benjamini and Hochberg, 1995). Besides calculating the correlations between age and all-night spectral power, we also computed these statistics for the NREM phase of sleep cycles 1–4 to investigate the potentially unequal contributions of different sleep cycles to subgroup differences.
3. Results
3.1. Sleep macrostructure
In the average intelligence (AIQ) subgroup, wakefulness after sleep onset (WASO) and relative N1 sleep duration correlated positively, while relative SWS duration correlated negatively with age. In the high intelligence (HIQ) subgroup, only WASO correlated positively with age while the other effects were not significant after correcting for multiple comparisons (see Table 2).
Table 2
Sleep architecture and its IQ level-dependent correlation with age. Correlations which are significant after correction for multiple comparisons are marked with an asterisk. The last two columns contain Fisher’s Z value for the comparison of the two corresponding correlation coefficient and the corresponding p-value, respectively. The comparison of correlation coefficients did not reach statistical significance after correcting for multiple comparisons.
3.2. NREM sleep EEG spectral power and age
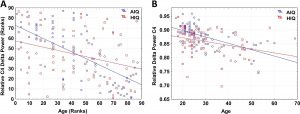
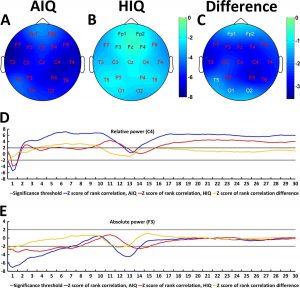
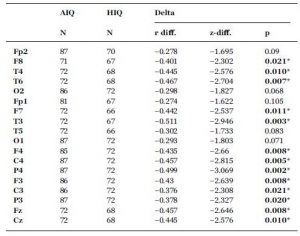
A negative association between age and relative NREM sleep EEG delta power was seen in both subgroups (Supplementary Table 1). Statistically significant differences between age-dependent changes in the sleep EEG spectral density of the AIQ and HIQ groups were present in case of relative delta: the HIQ group exhibited a smaller age-related loss of relative delta power (Fig. 2) with a maximum at the coronal midline (Fig. 3). After correction for multiple comparisons, the group differences in age-dependent decreases of relative NREM sleep EEG delta power remained significant on all except frontopolar and occipital derivations. Table 3 shows subgroup differences in the correlations of age vs. relative EEG delta power.
Fig. 2. Panel A: Scatterplot of the Spearman rank correlation of age and relative NREM delta power in AIQ (N=87) and HIQ (N=72) subjects on C4. Regression lines represent the Spearman rank correlation (defined as the Pearson correlation of ranks) of age and relative spectral power. Ranks in the HIQ group were multiplied by the NAIQ/NHIQ proportion in order to correct for the different rank range due to differences in subgroup size. Panel B: Scatterplot of absolute values of age and relative NREM delta power in AIQ (N=87) and HIQ (N=72) subjects on C4. Regression lines represent the Pearson correlation of age and relative spectral power (rAIQ=−0.282 rHIQ=−0.623, significantly different at Z=2.71, p < 0.001).
Fig. 3. Age-related changes of the all-night NREM sleep EEG spectrum. Topographical maps represent z-scores of Spearman correlation coefficients between age and relative NREM delta power in AIQ (panel A) and HIQ (panel B) subjects. Panel C shows topographical maps of the z-score differences between AIQ and HIQ subjects. The z-scores of the Spearman correlation coefficients between age and relative delta power on C4 (panel D) as well as absolute delta power on F3 (panel E) are also shown for each subgroup and each 0.25 Hz frequency bin included in the analysis. Red letters on panels A-C indicate derivations where correlations (panels A and B) or subgroup differences in the magnitude of the age-power correlation (panel C) were significant after correcting for multiple comparisons. Black or white electrode labels both indicate non-significant associations and were colored only to improve visibility.
Table 3
Subgroup differences (HIQ–AIQ) in the correlations between age and band-limited NREM sleep EEG relative delta power. r-difference indicates the difference of Fisher ztransformed correlation coefficients. z-difference indicates the normalized difference of Fisher z-transformed correlation coefficients (see Formula (3) for details). Asterisks indicate significant differences (Benjamini-Hochberg corrected p < 0.05) between the correlations of the two subgroups.
For absolute NREM sleep EEG power spectral density, a negative association between age and delta, theta and sigma log power was found (Supplementary Table 2.). The correlation between age and alpha/beta log power was not significant. The negative correlation between age and log delta power was smaller in the HIQ subgroup on F3 and F4 (uncorrected p-values 0.049 and 0.029, respectively). The negative correlation between age and log sigma power was weaker in the HIQ group on Fz (uncorrected p=0.045). The correlation between age and log alpha power on T6 was weakly positive in the AIQ subgroup and weakly negative in the HIQ subgroup, resulting in a significant difference (uncorrected p=0.048). These associations did not survive correcting for multiple comparisons. Other subgroup correlation differences did not reach statistical significance in case of log power, but the negative correlation coefficient between age and log power was weaker in the HIQ subgroup on all electrodes in case of the delta range and all electrodes except for T6, O1 and O2 in case of the sigma range.
Supplementary Fig. 1 presents subgroup differences in the association between age and EEG power with a higher resolution in the frequency domain.
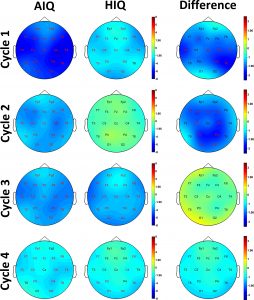
The correlation between age and logarithmized (absolute) as well as relative NREM delta power was strongest in the first sleep cycle, and diminished in further sleep cycles. Subgroup differences were also most prominently seen in the first sleep cycle, and almost completely gone by the 3rd and 4th cycles (see Supplementary Fig. 1 for details). In REM sleep, no group differences were significant after correcting for multiple comparisons.
4. Discussion
Alterations in sleep EEG oscillations were repeatedly hypothesized to play a crucial role in cognitive aging (Fogel et al., 2012; Dijk et al., 1989), with decreasing slow wave activity arguably being the most important age-related change in the sleep EEG. Our findings confirm ageing effects on sleep; however, this relationship is strongly modulated by general intelligence. When dividing our sample into individuals with average vs. high intelligence, we observed less age-related decline in objective indicators of sleep quality and a strikingly attenuated age effect on slow wave activity in the high intelligence subgroup.
NREM slow wave activity is an index of cortical thickness (Mander et al., 2013; Tamnes et al., 2010), therefore retained slow wave activity reflects retained cortical structure and function, and possibly more retained synaptic plasticity. The fact that slow wave activity was more retained in the HIQ subgroup suggests that age-related structural and functional changes in the cortex of these subjects are attenuated, potentially entailing a greater degree of cortical plasticity. This may result in a greater ability of HIQ subjects to retain their cognitive abilities to a higher age, resulting not only in better neuropsychological functioning, but potentially also enabling a more active lifestyle and facilitating better health behavior. Such an effect would be in line with previous results about more retained health in highly intelligent individuals (Batty et al., 2006, 2008, 2009), as well as studies which showed that sleep is a particularly strong index of neurocognitive and general health (Anderson and Horne, 2003; Mazzotti et al., 2014).
Combined, our findings suggest that high intelligence is associated with a lower degree of the detrimental effects of cerebral ageing, specifically concerning cortical thickness and plasticity which is reflected by NREM slow wave activity, and sleep EEG is an index of the degree of this effect. In line with reports about stronger age-related NREM sleep slow wave loss during the first half of the night (Carrier et al., 2001; Dijk et al., 1989), we found the strongest modulating effect of intelligence on the age-sleep association during the first two sleep cycles, suggesting that in ageing HIQ subjects the overnight course of slow wave activity indeed resembles that of younger subjects, instead of a compensation in later sleep cycles.
Importantly, effects were present both with absolute (log-transformed) and relative delta power. Individual variations in absolute EEG voltage due to the effects of skull and scalp thickness can influence absolute, but not relative power. Therefore, relative power is a more sensitive measure of individual differences in EEG spectral density, and it is unsurprising that the strongest subgroup differences emerged in measures of relative band power. However, one of the shortcomings of relative power is that slower frequencies always dominate the sleep EEG spectrum, and relative power measures in other frequency bands partially reflect inverted delta band effects. For this reason, our study of relative (normalized) power was limited to the delta band. In our methodology, normalization of spectral power was performed by two methods: first, by calculating the relative power across the frequency domain in order to remove individual differences in baseline spectral power and second, by also using sleep cycles-wise, effectively calculating power over a minor time interval. However, future studies may consider using other methods of normalization, such as normalizing spectral power across derivations.
Socioeconomic status (SES) is a potential confounding factor of our results. Higher SES is associated with better education, higher income and better overall health, including sleep quality (Moore et al., 2002; Lampert et al., 2013), likely mediating the effects of IQ even though we excluded subjects with serious sleep complaints and/or chronic illnesses and we did not have subjects with IQ scores below one standard deviation from the mean. Further studies may investigate the additional predictive power of IQ on sleep quality in ageing subjects beyond the effect of common health risk factors and/or SES, as it was shown before in case of malignant tumors and heart conditions(Batty et al., 2008, 2009). Another limitation of our study is its cross-sectional design: since IQ scores and sleep recordings were obtained from all subjects at the same age, we cannot reveal whether high performance in IQ tests is a cause or a consequence of retained NREM delta power in ageing subjects. Of further note is that volunteers were recruited also via Mensa Germany and Mensa Hungary to increase the number of highly intelligent individuals. Members of Mensa are aware of their intelligence and might be more aware of factors that contribute to healthy cognitive aging.
Overall, our results show that highly intelligent individuals show less prevalent age-related changes in their sleep EEG than average intelligence control subjects from a similar age range. These subgroup differences are apparent in sleep macrostructure, especially strong in the delta band of NREM sleep, which are the most prominently affected by ageing, but they are also present in higher frequencies. Although intelligence exhibits a large heritable component, interventions targeting age-related cognitive decline might thereby also ameliorate agerelated sleep impairments, which in turn might promote general mental health in the elderly.
Author contributions
AP, PPU, MD and RB designed research; AP, BNK, PS, MD and RB acquired data; AP, PPU and FG analyzed data; JK and FG provided tools for data analysis; all authors contributed to the writing and revision of the manuscript.
Acknowledgements
This study was supported by the Hungarian Medical Research Council (ETT-162/2003) and the Hungarian National Research Fund (OTKATS-049785), the Hungarian Brain Research Program (KTIA_NAP_13-1-2013-0001) as well as the general budgets of the Institute of Behavioural Sciences, Semmelweis University and the Max Planck Institute of Psychiatry. We would like to thank Mensa Germany and Mensa Hungary for their help in volunteer recruitment. BNK and MD are members of Mensa, however Mensa had no role in the study design, data analysis, data interpretation, or writing of the manuscript. All authors report no conflict of interest.
Appendix A. Supplementary material
References
Achermann, P., Dijk, D.-J., Brunner, D.P., Borbély, A.A., 1993. A model of human sleep homeostasis based on EEG slow-wave activity: quantitative comparison of data and simulations. Brain Res. Bull. 31 (1), 97–113.
Aeschbach, D., Borbely, A.A., 1993. All-night dynamics of the human sleep EEG. J. Sleep. Res. 2 (2), 70–81.
Anderson, C., Horne, J.A., 2003. Prefrontal cortex: links between low frequency delta EEG in sleep and neuropsychological performance in healthy, older people. Psychophysiology 40 (3), 349–357.
Batty, G.D., Der, G., Macintyre, S., Deary, I.J., 2006. Does IQ explain socioeconomic inequalities in health? Evidence from a population based cohort study in the west of Scotland.
Batty, G.D., Shipley, M.J., Mortensen, L.H., Gale, C.R., Deary, I.J., 2008. IQ in late adolescence/early adulthood, risk factors in middle-age and later coronary heart
disease mortality in men: the Vietnam Experience Study. Eur. J Cardiovasc. Prev.Rehabil. 15 (3), 359–361.
Batty, G.D., Kivimaki, M., Morrison, D., et al., 2009. Risk factors for pancreatic cancer mortality: extended follow-up of the original Whitehall Study. Cancer Epidemiol.
Biomarkers Prev. 18 (2), 673–675.
Benjamini, Y., Hochberg, Y., 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B (Methodol.), 289–300.
Borbely, A.A., Baumann, F., Brandeis, D., Strauch, I., Lehmann, D., 1981. Sleep deprivation: effect on sleep stages and EEG power density in man. Electroencephalogr. Clin. Neurophysiol. 51 (5), 483–495.
Brans, R.G., Kahn, R.S., Schnack, H.G., et al., 2010. Brain plasticity and intellectual ability are influenced by shared genes. J. Neurosci. 30 (16), 5519–5524.
Buchmann, A., Ringli, M., Kurth, S., et al., 2011. EEG sleep slow-wave activity as a mirror of cortical maturation. Cereb. Cortex 21 (3), 607–615.
Busby, K., Pivik, R.T., 1983. Sleep patterns in children of superior intelligence. J. Child Psychol. Psychiatry 24 (4), 587–600.
Carrier, J., Land, S., Buysse, D.J., Kupfer, D.J., Monk, T.H., 2001. The effects of age and gender on sleep EEG power spectral density in the middle years of life (ages 20–60 years old). Psychophysiology 38 (2), 232–242.
Cattell, R.B., 1973. Culture Fair Intelligence Test: (a measure of “g”). Institute for Personality and Ability Testing, Champaign, Ill.
Dijk, D.J., Beersma, D.G.M., van den Hoofdakker, R.H., 1989. All night spectral analysis of EEG sleep in young adult and middle-aged male subjects. Neurobiol. Aging 10 (6), 677–682.
Dresler, M., Spoormaker, V.I., Beitinger, P., et al., 2014. Neuroscience-driven discovery and development of sleep therapeutics. Pharmacol. Ther. 141 (3), 300–334.
Duncan, J., Seitz, R.J., Kolodny, J., et al., 2000. A neural basis for general intelligence. Science 289 (5478), 457–460.
Feinberg, I., Campbell, I.G., 2013. Longitudinal sleep EEG trajectories indicate complex patterns of adolescent brain maturation. Am. J Physiol. Regul. Integr. Comp. Physiol. 304 (4), 28.
Fogel, S., Martin, N., Lafortune, M., et al., 2012. NREM sleep oscillations and brain plasticity in aging. Front. Neurol. December 14;3.
Geiger, A., Achermann, P., Jenni, O.G., 2010. Association between sleep duration and intelligence scores in healthy children. Dev. Psychol. 46 (4), 949–954.
Hauser, R.M., 2002. Demography UoW–MCf, Ecology. Meritocracy, Cognitive Ability, and the Sources of Occupational Success. Center for Demography and Ecology, University of Wisconsin.
Hauser, R.M., Palloni, A., 2011. Adolescent IQ and survival in the Wisconsin longitudinal study. J. Gerontol. Ser. B: Psychol. Sci. Social. Sci. 66 (Suppl. 1), i91–i101.
Huber, R., Felice Ghilardi, M., Massimini, M., Tononi, G., 2004. Local sleep and learning. Nature 430 (6995), 78–81.
Huber, R., Ghilardi, M.F., Massimini, M., et al., 2006. Arm immobilization causes cortical plastic changes and locally decreases sleep slow wave activity. Nat. Neurosci. 9 (9), 1169–1176.
Iber, C., Ancoli-Israel, S., Chesson, A., Quan, S., 2007. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specification 1st ed.. American Academy of Sleep Medicine, Westchester, IL.
Kattler, H., Dijk, D.-J., BorbÉLy, A.A., 1994. Effect of unilateral somatosensory stimulation prior to sleep on the sleep EEG in humans. J. Sleep. Res. 3 (3), 159–164.
Kocevska, D., Rijlaarsdam, J., Ghassabian, A., et al., 2016. Early childhood sleep patterns and cognitive development at age 6 years: the generation R study. J. Pediatr. Psychol., 23.
Kurth, S., Ringli, M., Geiger, A., LeBourgeois, M., Jenni, O.G., Huber, R., 2010. Mapping of cortical activity in the first two decades of life: a high-density sleep electroencephalogram study. J. Neurosci. 30 (40), 13211–13219.
Kurth, S., Jenni, O.G., Riedner, B.A., Tononi, G., Carskadon, M.A., Huber, R., 2010. Characteristics of sleep slow waves in children and adolescents. Sleep 33 (4), 475.
Lampert, T., Kroll, L.E., von der Lippe, E., Muters, S., Stolzenberg, H., 2013. Socioeconomic status and health: results of the German Health Interview and Examination Survey for Adults (DEGS1). Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 56 (5–6), 814–821.
Mackintosh, N.J., 2011. IQ and Human Intelligence. Oxford University Press.
Mander, B.A., Rao, V., Lu, B., et al., 2013. Prefrontal atrophy, disrupted NREM slow waves and impaired hippocampal-dependent memory in aging. Nat. Neurosci. 16 (3), 357–364.
Mazzotti, D.R., Guindalini, C., Moraes, W., et al., 2014. Human longevity is associated with regular sleep patterns, maintenance of slow wave sleep and favorable lipid profile. Front. Aging Neurosci., 6.
Meng, X., D’Arcy, C., 2012. Education and dementia in the context of the cognitive reserve hypothesis: a systematic review with meta-analyses and qualitative analyses. PLoS One 7 (6), 4.
Moore, P.J., Adler, N.E., Williams, D.R., Jackson, J.S., 2002. Socioeconomic status and health: the role of sleep. Psychosom. Med. 64 (2), 337–344.
Oswald, W.D., Roth, E., 1987. Der Zahlen-Verbindungs-Test (ZVT). Ein sprachfreier Intelligenz-Test zur Messung der “kognitiven Leistungsgeschwindigkeit”. Handanweisung (2., überarbeitete und erweiterte Auflage). Göttingen: Hogrefe.
Prokosch, M.D., Yeo, R.A., Miller, G.F., 2005. Intelligence tests with higher g-loadings show higher correlations with body symmetry: Evidence for a general fitness factor mediated by developmental stability. Intelligence 33 (2), 203–213.
Raven, Raven, J., Court, J.H., 2004. Raven Haladó Progresszív Mátrixok Kézikönyv. Budapest: Edge2000 Kft.
Raz, N., 2000. Aging of the brain and its impact on cognitive performance: Integratio n of structural and functional findings. In: FIMCTA, Salthouse (Ed.), The Handbook of Aging and Cognition2nd ed. Lawrence Erlbaum Associates Publishers, Mahwah, NJ, US, 1–90.
Schmand, B., Smit, J.H., Geerlings, M.I., Lindeboom, J., 1997. The effects of intelligence and education on the development of dementia. A test of the brain reserve hypothesis. Psychol. Med. 27 (6), 1337–1344.
Tamnes, C.K., Østby, Y., Fjell, A.M., Westlye, L.T., Due-Tønnessen, P., Walhovd, K.B., 2010. Brain maturation in adolescence and young adulthood: regional age-related changes in cortical thickness and white matter volume and microstructure. Cereb.Cortex 20 (3), 534–548.
Thatcher, R.W., North, D., Biver, C., 2005. EEG and intelligence: relations between EEG coherence, EEG phase delay and power. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 116 (9), 2129–2141.
Thatcher, R.W., North, D., Biver, C., 2007. Intelligence and EEG current density using low-resolution electromagnetic tomography (LORETA). Hum. Brain Mapp. 28 (2), 118–133.
Tononi, G., Cirelli, C., 2014. Sleep and the price of plasticity: from synaptic and cellular homeostasis to memory consolidation and integration. Neuron 81 (1), 12–34.
Ujma, P.P., Konrad, B.N., Genzel, L., et al., 2014. Sleep spindles and intelligence: evidence for a sexual dimorphism. J. Neurosci. 34 (49), 16358–16368.
Williams, B.A., Pearlberg, S.L., 2006. Learning of three-term contingencies correlates with Raven scores, but not with measures of cognitive processing. Intelligence 34 (2), 177–191.
Yaffe, K., Falvey, C.M., Hoang, T., 2014. Connections between sleep and cognition in older adults. Lancet Neurol. 13 (10), 1017–1028.
Appendix A. Supplementary material