Research > Topics > Examination of electric activity of human hippocampus during REM sleep (Research performed at the Epilepsy Center, National Institute of Psychiatry and Neurology, Budapest Hungary, and coordinated by Prof. Péter Halász)
Because its considerable involvement in memory processes, the hippocampal formation situated in the medial part of the temporal lobes is an increasingly revealed area of brain research. A characteristic functional state of the hippocampus is indicated by hippocampal theta waves or hippocampal rhythmic slow activity (RSA), which can be observed with highest probability during active wakefulness and even more during REM sleep. The mechanisms of generation of hippocampal RSA in animals as well as the assumed functions of it were analysed on the level of single cell studies. Based on this studies complex neurobiological models of behaviour were developed, but due to methodological difficulties it is still unclear if there exists similar hippocampal RSA in humans. The few, anecdotic and controversial data which is available regarding this question come from studies characterized by common methodological problems which cannot resolve this issue. At the same time the function of the sleep-dependent hippocampal activity patterns, connected to the study of the relationship of hippocampus and memory became a hot topic.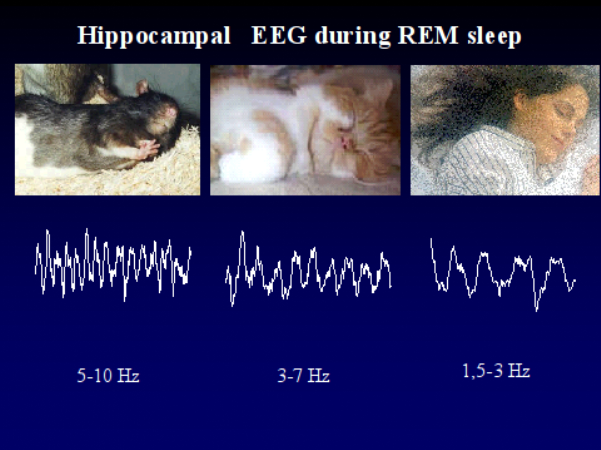 With this background we proposed to characterise the sleep-dependent activity patterns of the hippocampus in human subjects. On the other hand we hypothesised that there is a relationship between the individual-specific wake-sleep as well as sleep-phase-dependent activity patterns of the hippocampus and memory performances. In our studies we registered parahippocampal activity of epileptic patients undergoing presurgical evaluation with foramen ovale electrodes. This semi-invasive method makes it possible to register mediotemporal activity, which is hidden from the surface EEG methods. In contrast to earlier anecdotal reports based on similar approach our work relied on statistical analysis. Moreover we followed the methodological requirements, which were outlined in animal studies. The spectral analysis of electric activity patterns was based on Fourier transformation. The most important result of our study, which was not yet published by other research groups, is the observation of a 1.5-3 Hz rhythmic activity near the hippocampus. According to our results this activity is the correlate of human REM sleep and not of epilepsy-related pathology. We state that the hippocampal RSA is of 1.5-3 Hz, hence much slower in humans, than the well-known 5-9 Hz hippocampal theta in animals. The reason of that is not yet clear, but there are data available confirming that the ultra-fast 200 Hz hippocamapal ripples originally observed in rats are also slower, approximately of 80-160 Hz in humans. There is also a secondary 1 Hz spectral peak in the REM sleep data (outside the 1.5-3 Hz peak), which may build up from transient, irregular delta waves. With respect to the parahippocampal activity patterns there is a difference in dominant frequency between NREM and REM sleep. In contrast to the 1.5-3 Hz spectral peak of REM sleep, NREM sleep and especially the deep stages of it were characterized by a dominant peak of 1 Hz or somewhat lower than 1 Hz. This is in agreement with the cortical slow rhythm earlier described in animal and human studies, however it is the first report of its mediotemporal appearance in humans. In contrast to the 1.5-3 Hz activity the 1 Hz pattern appeared in the temporolateral scalp registered activity too. The spectral values of state-dependent and sleep phase-dependent activity patterns were correlated with the results of the standard neuropsychologic testing. The latter took place weeks before the electrophysiological recordings. Our aim was to find those activity patterns, which are the best predictors of efficient memory functioning in the neuroanatomical structures studied. The 1 Hz activity recorded from the right parahippocampal gyrus during deep NREM sleep correlated positively with short- and long-term visuo-spatial memory functioning according to the Rey-Osterrieth Complex Figure Test. The correlation between the left parahippocampal 1 Hz activity recorded during deep NREM sleep and the visuo-spatial memory performances was lower than that observed at the right side. The same activity recorded on the temporolateral surface (T3 and T4 scalp electrodes) correlated positively with visuo-spatial memory performances, but in contrast to the mediotemporal data there was no clear sign of cerebral laterality (both right and left-sided activity predicted memory performances). Age, years of intractable epilepsy and hippocampal MRI pathology (mostly hippocampal sclerosis) did not influence these relationships. The 1 Hz activity recorded during deep NREM sleep at the right anterior parahippocampal gyrus explained more than 68 percent of the intersubject variance in visuo-spatial memory. Our further result regarding the relationship between electrophysiological and cognitive variables is related to the phasic REM sleep (accompanied by eye movements) periods: the relative power of the secondary 1 Hz peak on the left side correlated positively with verbal learning ability. Similar to the above-mentioned correlations this one also could not be explained by other variables, such as age, years of pharmacoresistant epilepsy and hippocampal sclerosis. But this correlation is in accordance with the cerebral lateralization of verbal memory functions. Beyond the therapeutic application the parahippocampal electrocorticography performed with foramen ovale electrodes serves as an excellent tool for studying the hippocampus, sleep and memory in basic research. With this background we proposed to characterise the sleep-dependent activity patterns of the hippocampus in human subjects. On the other hand we hypothesised that there is a relationship between the individual-specific wake-sleep as well as sleep-phase-dependent activity patterns of the hippocampus and memory performances. In our studies we registered parahippocampal activity of epileptic patients undergoing presurgical evaluation with foramen ovale electrodes. This semi-invasive method makes it possible to register mediotemporal activity, which is hidden from the surface EEG methods. In contrast to earlier anecdotal reports based on similar approach our work relied on statistical analysis. Moreover we followed the methodological requirements, which were outlined in animal studies. The spectral analysis of electric activity patterns was based on Fourier transformation. The most important result of our study, which was not yet published by other research groups, is the observation of a 1.5-3 Hz rhythmic activity near the hippocampus. According to our results this activity is the correlate of human REM sleep and not of epilepsy-related pathology. We state that the hippocampal RSA is of 1.5-3 Hz, hence much slower in humans, than the well-known 5-9 Hz hippocampal theta in animals. The reason of that is not yet clear, but there are data available confirming that the ultra-fast 200 Hz hippocamapal ripples originally observed in rats are also slower, approximately of 80-160 Hz in humans. There is also a secondary 1 Hz spectral peak in the REM sleep data (outside the 1.5-3 Hz peak), which may build up from transient, irregular delta waves. With respect to the parahippocampal activity patterns there is a difference in dominant frequency between NREM and REM sleep. In contrast to the 1.5-3 Hz spectral peak of REM sleep, NREM sleep and especially the deep stages of it were characterized by a dominant peak of 1 Hz or somewhat lower than 1 Hz. This is in agreement with the cortical slow rhythm earlier described in animal and human studies, however it is the first report of its mediotemporal appearance in humans. In contrast to the 1.5-3 Hz activity the 1 Hz pattern appeared in the temporolateral scalp registered activity too. The spectral values of state-dependent and sleep phase-dependent activity patterns were correlated with the results of the standard neuropsychologic testing. The latter took place weeks before the electrophysiological recordings. Our aim was to find those activity patterns, which are the best predictors of efficient memory functioning in the neuroanatomical structures studied. The 1 Hz activity recorded from the right parahippocampal gyrus during deep NREM sleep correlated positively with short- and long-term visuo-spatial memory functioning according to the Rey-Osterrieth Complex Figure Test. The correlation between the left parahippocampal 1 Hz activity recorded during deep NREM sleep and the visuo-spatial memory performances was lower than that observed at the right side. The same activity recorded on the temporolateral surface (T3 and T4 scalp electrodes) correlated positively with visuo-spatial memory performances, but in contrast to the mediotemporal data there was no clear sign of cerebral laterality (both right and left-sided activity predicted memory performances). Age, years of intractable epilepsy and hippocampal MRI pathology (mostly hippocampal sclerosis) did not influence these relationships. The 1 Hz activity recorded during deep NREM sleep at the right anterior parahippocampal gyrus explained more than 68 percent of the intersubject variance in visuo-spatial memory. Our further result regarding the relationship between electrophysiological and cognitive variables is related to the phasic REM sleep (accompanied by eye movements) periods: the relative power of the secondary 1 Hz peak on the left side correlated positively with verbal learning ability. Similar to the above-mentioned correlations this one also could not be explained by other variables, such as age, years of pharmacoresistant epilepsy and hippocampal sclerosis. But this correlation is in accordance with the cerebral lateralization of verbal memory functions. Beyond the therapeutic application the parahippocampal electrocorticography performed with foramen ovale electrodes serves as an excellent tool for studying the hippocampus, sleep and memory in basic research.
References
- Bódizs R., Kántor S., Szabó G., Szűcs A., Erőss L., Halász P.: Rhythmic hippocampal slow oscillation characterizes REM sleep in humans, Hippocampus, 11(6), 747-753, 2001
- Bódizs R., Szűcs A., Halász P.: Does hippocampal theta exists in the human brain? [letter, comment], Neurobiology of Sleep-Wakefulness Cycle, 1(2), 102-105, 2001
- Bódizs R., Békésy M., Szűcs A., Barsi P., Halász P.: Sleep-dependent hippocampal slow activity correlates with waking memory performance in humans, Neurobiology of Learning and Memory, 78(2), 441-457, 2002
|
 With this background we proposed to characterise the sleep-dependent activity patterns of the hippocampus in human subjects. On the other hand we hypothesised that there is a relationship between the individual-specific wake-sleep as well as sleep-phase-dependent activity patterns of the hippocampus and memory performances. In our studies we registered parahippocampal activity of epileptic patients undergoing presurgical evaluation with foramen ovale electrodes. This semi-invasive method makes it possible to register mediotemporal activity, which is hidden from the surface EEG methods. In contrast to earlier anecdotal reports based on similar approach our work relied on statistical analysis. Moreover we followed the methodological requirements, which were outlined in animal studies. The spectral analysis of electric activity patterns was based on Fourier transformation. The most important result of our study, which was not yet published by other research groups, is the observation of a 1.5-3 Hz rhythmic activity near the hippocampus. According to our results this activity is the correlate of human REM sleep and not of epilepsy-related pathology. We state that the hippocampal RSA is of 1.5-3 Hz, hence much slower in humans, than the well-known 5-9 Hz hippocampal theta in animals. The reason of that is not yet clear, but there are data available confirming that the ultra-fast 200 Hz hippocamapal ripples originally observed in rats are also slower, approximately of 80-160 Hz in humans. There is also a secondary 1 Hz spectral peak in the REM sleep data (outside the 1.5-3 Hz peak), which may build up from transient, irregular delta waves. With respect to the parahippocampal activity patterns there is a difference in dominant frequency between NREM and REM sleep. In contrast to the 1.5-3 Hz spectral peak of REM sleep, NREM sleep and especially the deep stages of it were characterized by a dominant peak of 1 Hz or somewhat lower than 1 Hz. This is in agreement with the cortical slow rhythm earlier described in animal and human studies, however it is the first report of its mediotemporal appearance in humans. In contrast to the 1.5-3 Hz activity the 1 Hz pattern appeared in the temporolateral scalp registered activity too. The spectral values of state-dependent and sleep phase-dependent activity patterns were correlated with the results of the standard neuropsychologic testing. The latter took place weeks before the electrophysiological recordings. Our aim was to find those activity patterns, which are the best predictors of efficient memory functioning in the neuroanatomical structures studied. The 1 Hz activity recorded from the right parahippocampal gyrus during deep NREM sleep correlated positively with short- and long-term visuo-spatial memory functioning according to the Rey-Osterrieth Complex Figure Test. The correlation between the left parahippocampal 1 Hz activity recorded during deep NREM sleep and the visuo-spatial memory performances was lower than that observed at the right side. The same activity recorded on the temporolateral surface (T3 and T4 scalp electrodes) correlated positively with visuo-spatial memory performances, but in contrast to the mediotemporal data there was no clear sign of cerebral laterality (both right and left-sided activity predicted memory performances). Age, years of intractable epilepsy and hippocampal MRI pathology (mostly hippocampal sclerosis) did not influence these relationships. The 1 Hz activity recorded during deep NREM sleep at the right anterior parahippocampal gyrus explained more than 68 percent of the intersubject variance in visuo-spatial memory. Our further result regarding the relationship between electrophysiological and cognitive variables is related to the phasic REM sleep (accompanied by eye movements) periods: the relative power of the secondary 1 Hz peak on the left side correlated positively with verbal learning ability. Similar to the above-mentioned correlations this one also could not be explained by other variables, such as age, years of pharmacoresistant epilepsy and hippocampal sclerosis. But this correlation is in accordance with the cerebral lateralization of verbal memory functions. Beyond the therapeutic application the parahippocampal electrocorticography performed with foramen ovale electrodes serves as an excellent tool for studying the hippocampus, sleep and memory in basic research.
With this background we proposed to characterise the sleep-dependent activity patterns of the hippocampus in human subjects. On the other hand we hypothesised that there is a relationship between the individual-specific wake-sleep as well as sleep-phase-dependent activity patterns of the hippocampus and memory performances. In our studies we registered parahippocampal activity of epileptic patients undergoing presurgical evaluation with foramen ovale electrodes. This semi-invasive method makes it possible to register mediotemporal activity, which is hidden from the surface EEG methods. In contrast to earlier anecdotal reports based on similar approach our work relied on statistical analysis. Moreover we followed the methodological requirements, which were outlined in animal studies. The spectral analysis of electric activity patterns was based on Fourier transformation. The most important result of our study, which was not yet published by other research groups, is the observation of a 1.5-3 Hz rhythmic activity near the hippocampus. According to our results this activity is the correlate of human REM sleep and not of epilepsy-related pathology. We state that the hippocampal RSA is of 1.5-3 Hz, hence much slower in humans, than the well-known 5-9 Hz hippocampal theta in animals. The reason of that is not yet clear, but there are data available confirming that the ultra-fast 200 Hz hippocamapal ripples originally observed in rats are also slower, approximately of 80-160 Hz in humans. There is also a secondary 1 Hz spectral peak in the REM sleep data (outside the 1.5-3 Hz peak), which may build up from transient, irregular delta waves. With respect to the parahippocampal activity patterns there is a difference in dominant frequency between NREM and REM sleep. In contrast to the 1.5-3 Hz spectral peak of REM sleep, NREM sleep and especially the deep stages of it were characterized by a dominant peak of 1 Hz or somewhat lower than 1 Hz. This is in agreement with the cortical slow rhythm earlier described in animal and human studies, however it is the first report of its mediotemporal appearance in humans. In contrast to the 1.5-3 Hz activity the 1 Hz pattern appeared in the temporolateral scalp registered activity too. The spectral values of state-dependent and sleep phase-dependent activity patterns were correlated with the results of the standard neuropsychologic testing. The latter took place weeks before the electrophysiological recordings. Our aim was to find those activity patterns, which are the best predictors of efficient memory functioning in the neuroanatomical structures studied. The 1 Hz activity recorded from the right parahippocampal gyrus during deep NREM sleep correlated positively with short- and long-term visuo-spatial memory functioning according to the Rey-Osterrieth Complex Figure Test. The correlation between the left parahippocampal 1 Hz activity recorded during deep NREM sleep and the visuo-spatial memory performances was lower than that observed at the right side. The same activity recorded on the temporolateral surface (T3 and T4 scalp electrodes) correlated positively with visuo-spatial memory performances, but in contrast to the mediotemporal data there was no clear sign of cerebral laterality (both right and left-sided activity predicted memory performances). Age, years of intractable epilepsy and hippocampal MRI pathology (mostly hippocampal sclerosis) did not influence these relationships. The 1 Hz activity recorded during deep NREM sleep at the right anterior parahippocampal gyrus explained more than 68 percent of the intersubject variance in visuo-spatial memory. Our further result regarding the relationship between electrophysiological and cognitive variables is related to the phasic REM sleep (accompanied by eye movements) periods: the relative power of the secondary 1 Hz peak on the left side correlated positively with verbal learning ability. Similar to the above-mentioned correlations this one also could not be explained by other variables, such as age, years of pharmacoresistant epilepsy and hippocampal sclerosis. But this correlation is in accordance with the cerebral lateralization of verbal memory functions. Beyond the therapeutic application the parahippocampal electrocorticography performed with foramen ovale electrodes serves as an excellent tool for studying the hippocampus, sleep and memory in basic research.