|
*Epilepsy Center, National Institute of Psychiatry and Neurology, Hűvösvölgyi út 116, H-1021 Budapest, Hungary; †Neurotrend Ltd., Budapest, Hungary; and ‡Department of Radiology, National Institute of Psychiatry and Neurology, Budapest, Hungary Address correspondence and reprint request to Róbert Bódizs. Fax: 136-1-391-54-38.
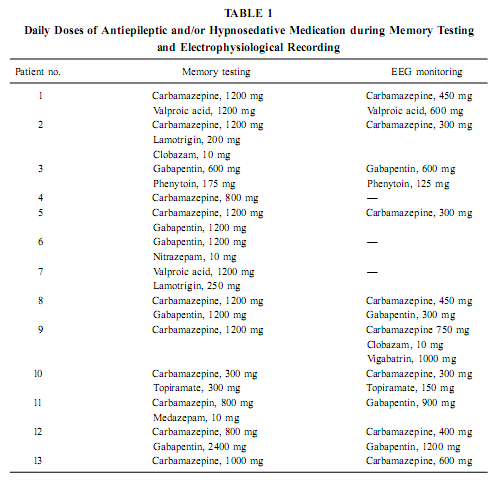
AbstractThe positive effect of postlearning sleep on memory consolidation as well as the relationship between sleep-related memory processes and the hippocampal formation are increasingly clarified topics in neurobiology. However, the possibility of a stable relationship between waking mnemonic performance and sleep-dependent hippocampal electric activity is unexplored. Here we report a correlative analysis between sleep-dependent parahippocampal–hippocampal (pHip-Hip) electric activity recorded by foramen ovale (FO) electrodes and different types of memory performances in epileptic patients. Psychological testing was performed days or weeks before electrophysiological recordings. The relative spectral power of the slow activity (below 1.25 Hz) during deep non-REM (NREM) sleep at the right pHip-Hip region correlated positively with the visual memory performance according to Rey–Osterrieth Complex Figure Test (ROCFT). Along the posterior–anterior direction of the hippocampal formation a linear increasing of correlations was observed. The relative power of the activity below 1.25 Hz at the left pHip-Hip during phasic REMsleep correlated positively with verbal learning performance and mnemonic retention values according to ROCFT. It is concluded that the pHip-Hip structures’ capacity of producing high amplitude and synchronized slow (< 1 Hz) oscillation during deep NREM sleep is related to the functional power of these structures.We hypothesize that the asymmetric (side-specific) propagation of ponto-geniculo-occipital (PGO) activity to the pHip-Hip region is related to the memory correlates of phasic REM sleep. KeyWords: electrocorticography; foramen ovale electrodes; sleep; NREM; REM; visual memory; Rey–Osterrieth Complex Figure Test; verbal learning; slow oscillation; EEG. INTRODUCTIONThe relationship between sleep and memory has been studied for decades (Jenkins & Dallenbach, 1924; McGrath & Cohen, 1978; Smith, 1995; Smith et al., 1998; Stickgold, 1998) and there is now convincing evidence for the positive effects of different stages of sleep on various forms of memory (Empson & Clarke, 1970; Karni et al., 1994; Plihal & Born, 1997, 1999; Gais et al., 2000; Wagner et al., 2001). The hippocampal formation is considered to be one of the main neuroanatomical structures orchestrating memory processing during sleep (Buzsáki, 1996; Wilson & McNaughton, 1994; McNaughton, 1998; Stickgold, 1998; Born & Fehm, 1998; Hasselmo, 1999; Louie & Wilson, 2001). Specific electrocortical sleep signs are presumed to be related to this sleep-dependent memory processing (Buzsáki, 1996; Sandyk, 1998; Poe et al., 2000; Steriade, 2000). The above-mentioned studies rely on the immediate effect of sleep on memory consolidation, while others emphasize traitlike relationships. For example, fast-learning rats have more REM sleep than slow-learning rats (Smith & Wong, 1991) and the increased REM sleep of the 5-HT1B receptor knock-out mice (Boutrel et al., 1999) is paralleled by an enhanced spatial memory performance in the Morris water maze (Malleret et al., 1999). Moreover, there is a positive correlation between IQ and certain REM sleep measures, particularly eye movement density, in mentally retarded children (Clausen et al., 1977; Pregram et al.,1986). The possibility that individual-specific (traitlike) hippocampal sleep signs are related to the level of waking memory functioning remained unexplored. In a recent study we found two prominent rhythmic components in the background EEG activity of human parahippocampal–hippocampal (pHip-Hip) structures during sleep: a <1 Hz oscillation characteristic for deep NREM sleep and a 1.50- to 3.00-Hz oscillation in REM sleep (Bódizs et al., 2001). In the present study we have correlated the power of pHip-Hip slow activities at different pHip-Hip locations with the results of standard neuropsychologic testing. By a similar approach Klimesch (1999) established a positive correlation between resting alpha frequency and mnemonic performance in normal human volunteers. Previous studies proved the beneficial effect of deep NREM sleep on explicit memory consolidation (Fowler et al., 1972; Plihal & Born, 1997; 1999), whereas REM sleep was shown to facilitate implicit memory (Karni et al., 1994; Smith, 1995; Plihal & Born, 1997; 1999). Explicit memory depends on the hippocampus, but implicit memory is independent of it (Squire & Zola-Morgan, 1991). Neural processes serving the consolidation of explicit memory traces are active during NREM sleep (Buzsa ´ki, 1996; Born & Fehm, 1998; Hasselmo, 1999). Given this relation it is reasonable to expect a correlation between NREM sleep pHip-Hip EEG measures and explicit memory performance. Based on the material-specific cerebral lateralization of memory functions (Bohbot et al., 1998; Breier et al., 1996; Golby et al., 2001), we hypothesize that verbal memory has a stronger correlation with left pHip-Hip EEG measures, whereas visual memory performances correlate more with right pHip-Hip EEG measures. This article describes a retrospective study investigating the relationship between pHip-Hip electrophysiologic activity patt MATERIALS AND METHODSSubjects Thirteen patients with a history of medically refractory epilepsy (four males and nine females, between ages 21 and 61 years, all right-handers) required mesiotemporal corticography to verify the location of a temporal lobe epileptogenic region for deciding surgical treatment. Four patients proved to have a right temporal, five patients a left temporal, and four patients a bitemporal epileptogenic region. According to the Raven test or the Hungarian version of the Wechsler Adult Intelligence Scale our patients’ intellect was normal (IQ within the range of 92–126, mean = 107.5). All patients finished elementary school (eight classes); two of them also finished secondary school. During memory testing patients were on their habitual antiepileptic medication, while during EEG monitoring a gradual decrease of antiepileptic medication was performed. For details of the treatment see Table 1.
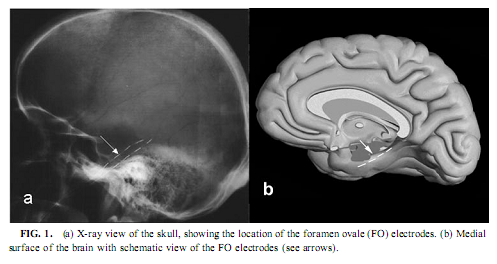
Magnetic Resonance Imaging Evaluation of the Patients Most of the patients were examined by the epilepsy MRI protocol published elsewhere (Barsi et al., 2000), the main feature of that being coronal T2 and proton density (PD)/ fluid attenuated inversion recovery (FLAIR) images perpendicular to the long axis of the hippocampus and multiplanar rapid acquisition gradient echo (MP-RAGE) 3D sequence with thin reconstructed slices in three directions.We assessed the presence of hippocampal sclerosis by the imaging symptoms of high T2 and PD/FLAIR signal intensity, low T1 signal intensity, blurred internal structure, and loss of volume. As we did not have the possibility to make volumetry, we assessed the extension of the pathologic signs in the diseased hippocampus by counting the slices with pathologic signs and multiplying those with the slice width plus interslice gap. We gave the measure of the diseased part of the hippocampus in the percentage of the whole size. Electrophysiological Recording Mesiotemporal corticography was performed with foramen ovale (FO) electrodes during presurgical video-EEG monitoring. Foramen ovale electrodes are flexible wires introduced through the foramen ovale into the cisterna ambiens and contact the parahippocampal gyrus at four points bilaterally along the axis of the hippocampal formation (Wieser et al., 1985; see Fig. 1). This kind of mesiotemporal corticography provides a unique opportunity to measure the hippocampal formation’s activity in a semi-invasive way. FO electrodes have four silver contacting points each 5 mm apart. Eleven of 13 recordings were bilateral. Additional electrodes of the 10-20 system and standard polysomnography were used to define sleep stages according to standard criteria (Rechtschaffen & Kales, 1968). Parallel scalp recordings of the 10-20 system, electro-oculography, submental electromyography, and electrocardiography were performed with Ag/AgCl electrodes fixed with collodium. X ray of the skull determined the location of the FO electrodes. Signals from all electrodes were prefiltered at 0.33 Hz, amplified, and digitized at 128 Hz with 12-bit resolution (Micromed BQ132 headbox). Depending on the clinical situation, patients spent 3–12 days in the monitoring room allowing sitting, lying, and sleeping. The patients were able to talk with visitors, watch TV, read, and so on. The timing of lights off was determined by the patients at will. Continuous video EEG monitoring was performed. A 5- to 10-min recording in the awake state with eyes open (without speaking and any major movement or watching TV) was stored for the waking-eyes open condition. Every night’s recording from lights off until spontaneous awakening was also stored on removable disks of 100-MB capacity (Iomega- ZIP). All other states were selected off-line from these stored data.
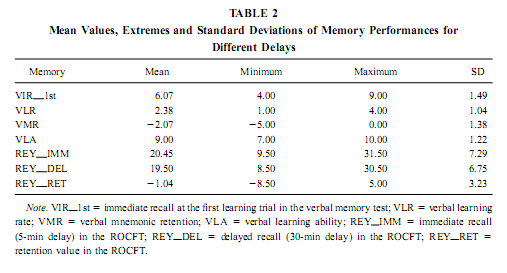
Quantitative EEG We have excluded the first night from our analysis and then selected representative samples of 4-s epochs for each patient’s sleep–waking state carefully avoiding artifacts and epileptic spikes in all the 32 simultaneously recorded channels. This was made visually according to Rechtschaffen and Kales (1968) criteria. Thirty to 100 epochs were selected for each patient’s sleep–waking state, which belonged to the recordings of night 2 or 3. The sleep–waking states defined here are the following: waking-eyes open, waking – eyes closed, light NREM sleep (Stage 2 sleep), deep NREM sleep (Stages 3 and 4 sleep), tonic REM periods (REM sleep periods without eye movements), and phasic REM periods (REM sleep periods accompanied by ocular motility). The standard reference point used in all patients was the vertex (Cz electrode). A Hanning window was applied for all selected epochs, after which they were subjected to fast Fourier transform, resulting in periodograms with 0.25-Hz spectral resolution. An average was then calculated for each sleep–waking state and each FO electrode of each subject. Absolute and relative power of the slow activity (< 1.25 Hz), as well as the mid-delta (1.50–3.00 Hz), high delta (3.25–4.50 Hz), low theta (4.75–6.25 Hz), mid-theta (6.50–7.75 Hz) and high theta or low alpha (8.00–9.50 Hz) bands, were calculated. As the major EEG activity below 1.25 Hz is the slow (0.7–1 Hz) activity (Steriade, 2000; Achermann and Borbély, 1997), expressing itself in spectral peaks of 0.75 or 1 Hz, the 0.33-Hz hardware prefilter do not affect the assessment of this oscillation. Neuropsychological Testing Neuropsychological testing was done days or weeks before the recording procedure. It was part of the preoperative neuropsychological investigation. In the verbal memory test the subjects had to learn 10 auditorily presented, common, Hungarian words during five learning trials. Every learning trial was followed by a free, immediate recall. Thirty minutes later the patients had to recall the words again. The interval of mnemonic retention was spent by performing other paper-and-pencil tests. The following measures of verbal memory were used: number of words recalled in the first learning trial, learning rate (number of words recalled in the fifth trial minus number of words recalled in the first learning trial), learning ability (number of words recalled in the fifth learning trial), and long-term retention (number of words recalled at 30 min delay minus number of words recalled in the fifth learning trial). The Rey–Osterrieth Complex Figure Test (ROCFT) was used to assess visuospatial memory (Meyers & Meyers, 1995). While performing this test, the subjects had to copy a complex figure consisting of various geometrical components. The test instruction contained no hints to memorize the figure. The subjects had to redraw the figure 5 min and 30 min later relying entirely on visual memory. Based on the 18-item (36-point) scoring system (Meyers & Meyers, 1995) three indices of visual memory were used in this study: immediate recall (performance at 5-min delay), delayed recall (30-min delay), and retention (delayedminus immediate). Many clinical and research applications of the ROCFT use an immediate recall instead of a 5-min delay, for the first recall trial. In our practice the copy trial of the ROCFT is followed by a handedness inventory (Edinburgh or Annett inventories), which serves also as a distraction and usually takes 3–5 min.We prefer this kind of administration because of greater long-term memory load and for avoiding contamination between short- and long-term memory in the immediate recall trial. For assesing the general cognitive abilities of the patients we used the Raven test or the Hungarian version of the Wechsler Adult Intelligence Scale. Statistical Analysis Missing data in the two cases of unilateral recording were RESULTS AND DISCUSSIONVerbal and Visual Memory Performances Mean performance measures of verbal and visual memory for different delays as well as their minimum, maximum and standard deviation are presented in Table 2.
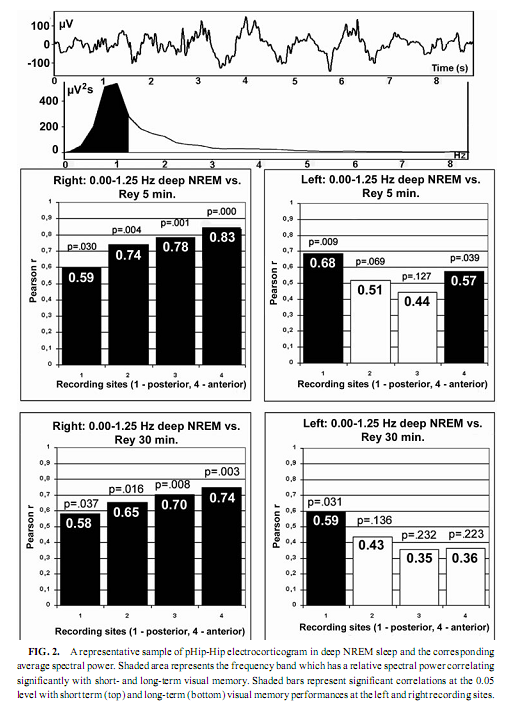
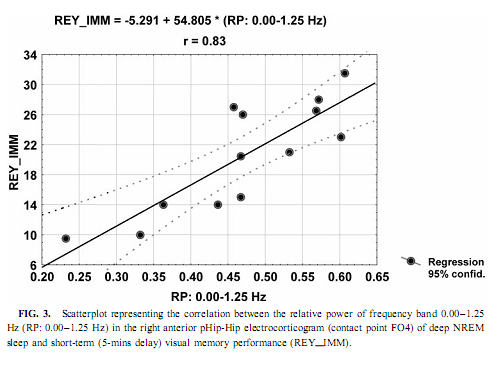
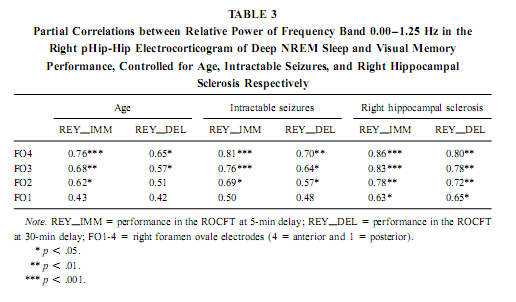
NREM Sleep pHip-Hip EEG and Memory Spectral frequency values in waking and light NREM sleep (Stage 2) do not correlate with memory performance. The relative power of the electrocortical activity below 1.25 Hz at the right side during deep NREM sleep (Stages 3 and 4), however, showed a significant positive correlation with short- (5 min) and long- (30-min) term visual memory (Figs. 2 and 3). Visual inspection revealed differences in the strength of correlations, but these differences did not reach the level of statistical significance. The correlations with short-term memory values were higher than with long-term values. Furthermore, along the posterior–anterior axis of the hippocampal formation a linear increase of correlations was observed. Weaker correlations were at the left side, and they reached the level of statistical significance in only two recording points (Fig. 2). Although the number of subjects is low (N = 13), the correlations were not driven by a few extreme cases (Fig. 3). Partialing out the effect of age, years of intractable seizures, and hippocampal sclerosis, respectively, has no effect other than slightly reducing the correlations (Table 3.). The slow frequency range below 1.25 Hz comprised a clear-cut oscillation of < 1 Hz (see Fig. 2).
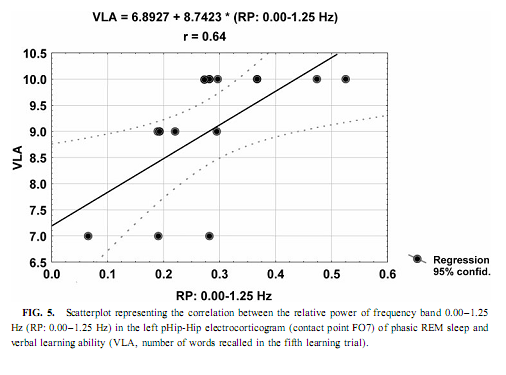
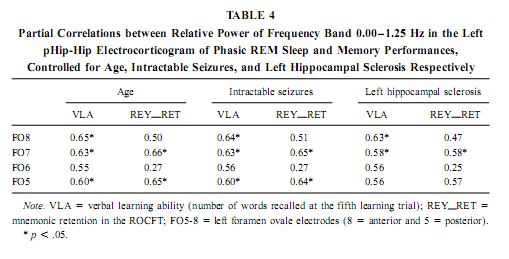
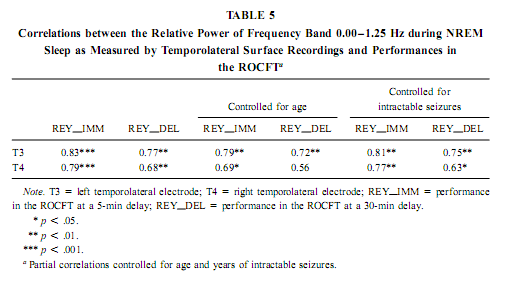
The relative power of the frequency bands above 1.50 Hz during deep NREM sleep correlate negatively with short- and long-term visual memory performances, but the statistical control of age, years of intractable seizures, and hippocampal sclerosis respectively reduced the correlation below the level of statistical significance. REM Sleep pHip-Hip EEG and Memory The relative power of the electrocorticographic activity below 1.25 Hz at the left side during phasic but not tonic REM sleep (i.e., periods accompanied by eye movement bursts) showed a positive correlation with verbal learning ability (number of words recalled at the fifth learning trial) and visual memory retention in the ROCFT (Figs. 4 and 5). REM sleep is characterized by a continuous, synchronous, and coherent 1.50- to 3.00- Hz oscillatory activity,which is the frequency range of REMsleep-specific human rhythmic hippocampal slow activity (Bódizs et al., 2001). The secondary frequency peak around 1 Hz consists of transient, nonrhythmic, irregular slow waves. Partialing out the effect of age and that of intractable seizures does not affect the correlation between the left-sided phasic REM-dependent pHip-Hip < 1.25-Hz relative power and verbal learning. Controlling for left hippocampal sclerosis slightly reduces the correlations, with no other effect (Table 4). The correlation between the relative power of the electrocorticographic activity below 1.25 Hz and verbal learning is limited to the phasic REM periods. It is reasonable to test the difference between phasic and tonic REMsleep regarding the relative power of this activity. Using all contact points (eight per patient) as statistical units, the electrocorticographic activity below 1.25 Hz has significantly higher relative power in phasic REM sleep than in tonic REM sleep (F = 21.38; df = 1, 123; p < .00001). Temporolateral EEG and Memory Temporolateral surface activity showed the same spectral peak of 0.75 or 1 Hz during deep NREM sleep as we have found in FO recordings (see Bo ´dizs et al., 2001). The correlation between the relative power of the slow activities below 1.25 Hz on the temporolateral surface EEG in deep NREM sleep and the performance in the ROCFT was also found. In contrast to the correlations found in the case of the FO electrodes the temporolaterally found correlations showed no clear-cut sign of laterality (Table 5). Almost all of the correlations remained significant after the statistical control of age and years of intractable seizures, but this latter procedure resulted in higher correlations at the left temporolateral recording site. The Significance of the Correlative Approach We have studied the relationship between state-dependent pHip-Hip EEG features and memory performances. Of course these relationships do not reflect the immediate effect of sleep on memory because the memory testing sessions preceded electrophysiological recordings by days or weeks. The relationships reported here reflect a general correlation between sleep-dependent mediotemporal neural processes and memory. The hippocampus plays an essential role in memory (Squire & Zola-Morgan, 1991). This is supported by structural and functional data. An example for structural evidence is the correlation between the level of hippocampal atrophy as measured by MRI and memory performance (Baxendale, 1995; Kopelman et al., 2001). Functional measures of hippocampal memory processing are given in PET and fMRI studies (Gabrieli et al., 1998; Yasuno et al., 1999; Kohler et al., 2000; Golby et al., 2001). Sleep is supposed to be a state of memory reprocessing (McNaughton, 1998; Stickgold, 1998), hence electrophysiological parameters of sleep-dependent pHip-Hip activity could be a sensitive measure of waking memory functioning.
In previous sleep and memory studies, memory was regarded as a plastic function, which can be influenced by various circumstances, including different sleep stages. The obvious differences that exist in mnemonic capacity of different persons was not taken into account. Memory is also a traitlike phenomenon. By accepting this statement Klimesch (1999) found a positive correlation between resting alpha frequency and memory performance. In our study we followed this latter approach.
Slow (<1 Hz) Oscillation, NREM Sleep, and Cerebral Lateralization of Visual Memory The results of the present study partially confirmed our hypotheses. The relative power of the electrocortical activity below 1.25 Hz in deep NREM sleep correlated positively with short- and long-term visual memory performances. The right anterior mediotemporal region showed the strongest correlations, which is in accordance with much, but not all, data regarding cerebral lateralization and neuropsychologic localization of visual memory functions, including performances in the ROCFT (Roland & Gulyas, 1995; Bohbot et al., 1998; Breier et al., 1996; Barr et al., 1997). The right-sided NREM sleep pHip-Hip EEG, however, did n At the temporolateral surface recordings significant correlations between the deep NREM sleep <1.25 Hz relative power and the ROCFT did not show the right-sided predominance observed in the FO recordings. This means that the cerebral lateralization of the ROCFT applies only to the mediotemporal structures in our data. The temporolateral cortex, although strongly related to the performance in the ROCFT, shows no unambiguous signs of lateralization. The electrocorticographic and EEG activity below 1.25 Hz comprised a clear-cut oscillation of <1 Hz, which reflects a general functional property of cortical neurons during NREM sleep in animals and humans (Steriade & Amzica, 1998; Achermann & Borbély, 1997). It was outlined that the slow oscillation of this frequency consists of synchronized, rhythmic depolarizations followed by long-lasting hyperpolarizations in cortical neurons during NREM sleep. The phases of depolarizations are characterized by intensive neural firing (Steriade & Amzica, 1998; Steriade, 2000). Deep NREM sleep differs from the light sleep in the widespread synchronization and general acceleration of this slow rhythm (Steriade & Amzica, 1998). Our results are in agreement with the hypothesis of Steriade (2000), who supposes that the slow oscillation during NREM sleep facilitates reorganization processes in corticothalamic and corticocortical circuitry leading to enhanced neural plasticity. We suggest that elements of the neural circuitry responsible for the generation of the slow oscillation during deep NREM sleep are shared by mnemonic systems implicated in the performance in the ROCFT. Irregular Slow Activity, REM Sleep, and Cerebral Lateralization of Verbal Learning Ability In contrast to our hypotheses the relative power of the electrocorticographic activity below 1.25 Hz at the left-side pHip-Hip structures during phasic REM sleep correlated positively with verbal learning ability. The solely left-sided correlations rule out the possibility that this activity reflects eye movement artifacts during phasic REM sleep. The correlation of verbal memory and hippocampal activity on the left side is in accordance with the neuropsychologic data regarding the cerebral lateralization of verbal memory functions (Rausch& Babb, 1993; Golby et al., 2001). As the correlations appeared only with phasic REM sleep EEG, and phasic REM sleep is richer in PGO activity than tonic REM sleep, we can only speculate that the irregular 1-Hz frequency component reflects the PGO activity. This is a major electrophysiologic feature of REM sleep and it tends to accompany eye movement bursts (Vanni-Mercier et al., 1994). This is in accordance with our finding that the < 1.25-Hz relative power is significantly higher during phasic than during tonic REM sleep. It was recently proven that PGO activity—arising in the pons—has major output to the main memory systems of the brain, including the hippocampal formation and the entorhinal cortex, and the PGO activity correlates positively with avoidance learning in rats (Datta, 2000). Different Cerebral Lateralization of Visual Memory Performance and Retention The functional significance of the left-sided electrocorticographic activity below 1.25 Hz during phasic REM sleep is supported by the significant positive correlations between the relative spectral power of this activity during phasic REM sleep and the ROCFT retention values (5-min delay values were substracted from the 30-min delay values). Interestingly this result suggests that the mnemonic retention of the Rey–Osterrieth Complex figure depends on the functional properties of the left hippocampal formation. This could mean a functional dissociation of abstract visual memory from the organizational processes acting on this visual material during the retention interval. While absolute indices of short- and long-term visual remembering are related to the right hippocampal formation (Figs. 2 and 3), individualized relative measures expressing the difference between long- and short-term values tend to correlate with left hippocampal functioning (Fig. 4). Possible Confounding Variables Age, years of intractable seizures, and the level of hippocampal sclerosis had no substantial influence on the correlations. However, all patients in this study were temporal lobe epileptics and there is no control group, thus the possibility that the correlations are influenced by other pathologic phenomena cannot entirely be ruled out. This limits the extent to which findings can be generalized. Replicating this finding on a greater number of unequivocally unilateral temporal epileptic patients or on patients suffering from extra- temporal epilepsy could confirm these relationships. It must be mentioned that these kinds of recordings are made only in epileptic patients, therefore having an appropriate control group is practically excluded. There was no difference in the ROCFT performance of the left, right, and bitemporal subgroups (F = 0.63; df = 2, 10; p = 0.54 for immediate recall and F = 3.33; df = 2, 10; p = 0.07 for delayed recall). The tendency for significant difference is very low (practically zero) in the case of immediate recall and somewhat higher in delayed recall performance. The latter is due to the lower delayed recall values of left temporal epileptic patients. Verbal learning ability, however, differed significantly between left, right, and bitemporal epileptic subgroups (F = 9.28; df = 2, 10; p < .006). The difference is caused by the low verbal learning ability of left temporal epileptic patients as shown by the post hoc Scheffé test ( p < .02 for both comparisons). These results suggest that left temporal epileptic patients have lower performance in the delayed recall of the complex figure and the verbal learning ability. However, these results do not disprove the correlation between pHip-Hip EEG and memory because only one of these measures (verbal learning ability) correlated with the left pHip-Hip EEG, while the other (delayed ROCFT) was related to the right mediotemporal structure’s activity. Both of them were controlled for hippocampal sclerosis. Moreover, the strongest correlation was found between the immediate recall performance in the ROCFT and deep NREM sleep’s right pHip-Hip slow activity. The immediate recall performance in the ROCFT did not differ between the epilepsy subgroups and more than 68% of its interindividual variability was explained by the deep NREM sleep-related slow activity of the most anterior right FO electrode. All of our patients were on their habitual antiepileptic medication during memory testing, thus some negative cognitive effects cannot be excluded. A few issues pertain to the ROCFT. This is not a pure test of visuospatial memory and involves multiple cognitive domains. We used the classical 18-item scoring system in our study originally introduced by Osterrieth and it is probably the most popular form of clinical scoring. Visuospatial constructional abilities as measured by performance in the copy trial were not addressed in our study, but they could influence the memory performances. Using different scoring systems may help parse out some of the above-mentioned effects. CONCLUSIONSSummarizing our results we can conclude that sleep-dependent, phase-specific hippocampal slow activity is a good marker of waking memory functioning. During deep NREM sleep the synchronized slow (<1-Hz) oscillation of cortical origin is an exceptionally reliable correlate of the right hippocampus-dependent visual memory performance. More- over, the pattern of correlation coefficients shows anterior hippocampal predominance in the localization of this memory function. which is in accordance with current neuropsychological knowledge (Roland & Gulyas, 1995; Bohbot et al., 1998; Breier et al., 1996). It can be hypothesized that the right pHip-Hip structures’ capacity of producing The relation of phasic REM sleep and cognition was emphasized in several studies using mentally retarded children (Clausen et al., 1977; Pregram et al., 1986) or difficult learning situations in healthy volunteers (Smith & Lapp, 1991; Mandai et al., 1989). Here we show that left pHip-Hip electric activity below 1.25 Hz during phasic REM sleep is related to verbal learning ability and long-term mnemonic retention indices of an abstract figure. There are proven asymetries in the PGO activity (Monaco et al., 1984) and recent evidences suggest a relation between PGO waves and memory (Datta, 2000). These facts led us to hypothesize that the < 1.25-Hz activity during phasic REMsleep is an expression of the irradiation of PGO waves to the pHip-Hip region, which results in efficient waking memory functioning.
ReferencesAchermann, P., & Borbély, A. A. (1997). Low-frequency (, 1 Hz) oscillations in the human sleep electroencephalogram. Neuroscience, 81, 213–222. Barr,W. B., Chelune, G. J., Hermann, B. P., Loring, D.W., Perring, K., Strauss, E., Trenerry M. R., &Westerveld, M. (1997). The use of figural reproduction tests as measures of nonverbal memory in epilepsy surgery candidates. Journal of the International Neuropsychological Society, 3, 435–443. Barsi, P., Kenéz, J., Solymosi, D., Kulin, A., Halász, P., Rásonyi, G., Janszky, J., Kaloczkai, A., Barcs, G., Neuwirth, M., Paraicz, E., Siegler, Z., Morvai, M., Jerney, J., Kassay, M., & Altmann, A. (2000). Hippocampal malrotation with normal corpus callosum: A new entity? Neuroradiology, 42, 339–345. Baxendale, S. A. (1995). The hippocampus: Functional and structural correlations. Seizure, 4, 105–117. Bódizs, R., Kántor, S., Szabó, G., Szűcs, A., Erőss, L., & Halász, P. (2001). Rhythmic hippocampal slow oscillation characterizes REM sleep in humans. Hippocampus, 11, 747–753. Bohbot,V.D.,Kalina,M., Stepankova,K., Spackova,N., Petrides,M.,&Nadel, L. (1998). Spatial memory deficits in patients with lesion the right hippocampus and to the right parahippocampal cortex. Neuropsychologia, 36, 1217–1238. Born, J., & Fehm, H. L. (1998). Hypothalamus-pituitary-adrenal activity during human sleep: A coordinating role for the limbic hippocampal system. Experimental and Clinical Endocrinology and Diabetes, 106, 153–163. Boutrel, B., Franc, B., Hen, R., Hamon, M., & Adrien, J. (1999). Key role of 5-HT1B receptors in the regulation of paradoxical sleep as evidenced in 5-HT1B knock-out mice. Journal of Neuroscience, 19, 3204–3212. Breier, J. I., Plenger, P. M., Castillo, R., Fuchs, K., Wheless, J. W., Thomas, A. B., Brookshire, B. L., Willmore, L. J., & Papanicolao, A. (1996). Effects of temporal lobe epilepsy on spatial and figural aspects of memory for a complex geometric figure. Journal of the International Neuropsychological Society, 2, 535–540. Buzsáki, G. (1996). The hippocampo-neocortical dialogue. Cerebral Cortex, 6, 81–92. Empson, J. A., & Clarke, P. R. (1970). Rapid eye movements and remembering. Nature, 227, 287–288. Clausen, J., Sersen, E. A., & Lidsky, A. (1977). Sleep patterns in mental retardation: Down’s syndrome. Electroencephalography and Clinical Neurophysiology, 43, 183–191. Datta, S. (2000). Avoidance task training potentiates phasic pontine-wave density in the rat: A mechanism for sleep-dependent plasticity. Journal of Neuroscience, 20, 8607–8613. Fowler, M. J., Sullivan, M. G., & Ekstrand, B. R. (1972). Sleep and memory. Science, 179, 302–4. Gabrieli, J. D., Brewer, J. B., & Poldrack, R. A. (1998). Images of medial temporal lobe functions in human learning and memory. Neurobiology of Learning and Memory, 70, 275–283, doi:10.1006/nlme.1998.3853. Gais, S., Plihal, W., Wagner, U., & Born, J. (2000). Early sleep triggers memory for early visual discrimination skills. Nature Neuroscience, 3, 1335–1339. Golby, A. J., Poldrack, R. A., Brewer, J. B., Spencer, D., Desmond, J. E., Aron, A. P., & Gabrieli, D. E. (2001). Material-specific lateralization in the medial temporal lobe and prefrontal cortex during memory encoding. Brain, 124, 1841–1854. Hasselmo, M. E. (1999). Neuromodulation: Acetylcholine and memory consolidation. Trends in Cognitive Sciences, 3, 351–359. Jenkins, J. G., & Dallenbach, K. M. (1924). Oblivescence during sleep and waking. American Journal of Psychology, 35, 605–612. Karni, A., Tanne, D., Rubenstein, B. S., Askenasy, J. J., & Sagi, D. (1994). Dependence on REM sleep of overnight improvement of a perceptual skill. Science, 265, 679–682. Klimesch, W. (1999). EEG alpha and theta oscillations reflect cognitive and memory performance: A review and analysis. Brain Research Reviews, 29, 169–195. Kohler, S.,Moscovitch,M.,Winocur, G.,&McIntosh,A. R. (2000). Episodic encoding and recognition of pictures and words: Role of the human medial temporal lobes. Acta Psychologica (Amsterdam), 105, 159–179. Kopelman, M. D., Lasserson, D., Kingsley, D., Bello, F., Rush, C., Stanhope, N., Stevens, T., Goodman, G., Heilpern, G., Kendall, B., & Colchester, A. (2001). Structural MRI volumetric analysis in patients with organic amnesia. 2. Correlations with anterograde memory and executive tests in 40 patients. Journal of Neurology, Neurosurgery and Psychiatry, 71, 23–28. Louie, K., & Wilson, M. A. (2001). Temporally structured replay of awake hippocampal ensemble activity during rapid eye movement sleep. Neuron, 29, 145–156. Malleret, G., Hen, R., Guillou, J. L., Segu, L., & Buhot, M. C. (1999). 5-HT1B receptor knock-out mice exhibit increased exploratory activity and enhanced spatial memory performance in the Morris water maze. Journal of Neuroscience, 19, 6157–6168. Mandai, O., Guerrien, A., Sockeel, P., Dujardin, K., & Leconte, P. (1989). REM sleep modifications following a Morse code learning session in humans. Physiology and Behavior, 46, 639–642. McGrath, M. J., & Cohen, D. B. (1978). REM sleep facilitation of adaptive waking behavior: A review of the literature. Psychological Bulletin, 85, 24–57. McNaughton, B. L. (1998). The neurophysiology of reminescence. Neurobiology of Learning and Memory, 70, 252–267, doi:10.1006/nlme.1998.3851. Meyers, J. E., & Meyers, K. R. (1995). Rey Complex Figure Test and Recognition Trial: Professional Manual. Odessa: Psychological Assesment Resources. Monaco, A. P., Baghdoyan, H. A., Nelson, J. P., & Hobson, J. A. (1984). Cortical wave amplitude and eye movement direction are correlated in REM sleep but not in waking. Archives Italiennes de Biologie, 122, 213–223. Plihal, W., & Born, J. (1997). Effects of early and late nocturnal sleep on declarative and procedural memory. Journal of Cognitive Neuroscience, 9, 534–537. Plihal, W., & Born, J. (1999). Effects of early and late nocturnal sleep on priming and spatial memory. Psychophysiology, 36, 571–582. Poe, G. R., Nitz, D. A., McNaughton, B. L., & Barnes, C. A. (2000). Experience-dependent phase-reversal of hippocampal neuron firing during REM sleep. Brain Research, 855, 176–180. Pregram, G. V., Connell, B. E., Gnadt, J., & Weiler, D. (1986). Neuropsychology and the field of sleep and sleep disorders. In S. B. Filskov & J. B. Boll (Eds.), Handbook of clinical neuropsychology (pp. 426–492). New Work: Wiley. Rausch, R., & Babb, T. L. (1993). Hippocampal neuron loss and memory scores before and after temporal lobe surgery for epilepsy. Archives of Neurology, 50, 812–817. Rechtschaffen, A., & Kales, A. (eds) (1968). A manual of standardize Roland, P. E., & Gulyas, P. (1995). Visual memory, visual imagery, and visual recognition of large field patterns by the human brain: Functional anatomy by positron emission tomography. Cerebral Cortex, 5, 79–93. Sandyk, R. (1998). A neuromagnetic view of hippocampal memory functions. International Journal of Neuro- science, 93, 251–256. Smith, C. (1995). Sleep states and memory processes. Behavioural Brain Research, 69, 137–145. Smith, C., & Lapp, L. (1991). Increases in number of REMS and REM density in humans following an intensive learning period. Sleep, 14, 325–330. Smith, C., & Wong, P. T. (1991). Paradoxical sleep increases predict succesful learning in a complex operant task. Behavioral Neuroscience, 105, 282–288. Smith, C. T., Conway, J. M., & Rose, G. M. (1998). Brief paradoxical sleep deprivation impairs reference, but not working, memory in the radial arm maze task. Neurobiology of Learning and Memory, 69, 211–217, doi:10.1006/nlme.1997.3809. Squire, L. R., & Zola-Morgan, S. (1991). The medial temporal lobe memory system. Science, 253, 1380–1386. Steriade, M. (2000). Neuronal substrates for short-term plasticity during sleep oscillations in corticothalamic networks. In A. A. Borbély, O. Hayaishi, T. J. Sejnowski, and J. S. Altman (Eds.), The regulation of sleep (pp. 30–44). Strasbourg: HFSP. Steriade, M., & Amzica, F. (1998). Coalescence of sleep rhythms and their chronology in corticothalamic networks. Sleep Research Online, 1, 1–10. Stickgold, R. (1998). Sleep: Off-line memory reprocessing. Trends in Cognitive Sciences, 2, 484–492. Vanni-Mercier, G., Pelisson, D., Goffart, L., Sakai, K., & Jouvet, M. (1994). Eye saccade dynamics during paradoxical sleep in the cat. European Journal of Neuroscience, 6, 1298–1306. Wagner, U., Gais, S., & Born, J. (2001). Emotional memory formation is enhanced across sleep intervals with high amounts of rapid eye movement sleep. Learning and Memory, 8, 112–119. Wieser, H. G., Elger, C. E., & Stodieck, S. R. G. (1985). The ‘foramen ovale electrode’: A new recording method for the preoperative evaluation of patients suffering from mesio-basal temporal lobe epilepsy. Electroencephalography and Clinical Neurophysiology, 61, 314–322. Wilson, M. A., & McNaughton, B. L. (1994). Reactivation of hippocampal ensemble memories during sleep. Science, 265, 676–679. Yasuno, F., Nishikawa, T., Tokunaga, H., Nakagawa, Y., Ikejiri, Y., Oku, N., Hashikawa, K., Tanabe, H., Sugita, Y., Nishimura, T., & Takeda, M. (1999). Perception and conception: Separate memory systems in the medial temporal lobe. Methods and Findings in Experimental and Clinical Pharmacology, 21, 511–514.
|