Intelligence 72 (2019) 13-19. DOI: 10.1016/j.intell.2018.11.004
Pesonen Anu-Katriina a, Ujma Péter b, Halonen Risto a, Räikkönen Katri a, Kuula Liisa a
a Department of Psychology and Logopedics, Faculty of Medicine, University of Helsinki, Finland
b Semmelweis University, Institute of Behavioural Sciences, Budapest, Hungary
Abstract
Sleep spindles are EEG oscillations in NREM sleep which are implicated in sleep-related cerebral plasticity. Several studies indicate that sleep spindle characteristics are correlates of general cognitive ability with a possible sexual dimorphism, but most of the literature consists of small studies with heterogeneous results. In order to clarify these findings, we conducted the largest study into this topic (N = 176) in a birth cohort of 17 year old adolescents using all-night polysomnography and two different sleep spindle detectors. We failed to find significant associations between sleep spindle parameters and general cognitive ability. There was no evidence for a sexual dimorphism. Our findings suggest that previous small studies with limited statistical power and probability to sampling error may have detected sporadic associations which were not confirmed in this study. Large studies and meta-analyses, still somewhat uncommon in the quantitative EEG studies of psychological phenotypes, are necessary to evaluate the existing literature.
Keywords: IQ; General cognitive ability; Sleep spindle; NREM sleep; Electroencephalography
1. Introduction
Sleep spindles are prominent electroencephalographic oscillations which appear in non-rapid eye movement (NREM) sleep with a frontal and centroparietal midline maximum (Fogel & Smith, 2011; Urakami, Ioannides, & Kostopoulos, 2012). They arise as a result of the interaction of thalamocortical and corticothalamic neurons, driven by a reticular thalamic “pacemaker” (Lüthi, 2014; Steriade, 2003; Urakami et al., 2012), and are thought to play an important role in driving synaptic plasticity in NREM sleep (Lüthi, 2014; Rasch & Born, 2013). This includes long-term potentiation (LTP) (Rosanova & Ulrich, 2005), which is otherwise usually absent from NREM sleep. Due to their role in plasticity, sleep spindles have been extensively studied for their proposed role in the behavioral manifestation of synaptic plasticity: learning. Early studies indicated a general positive association between the presence of sleep spindles and the success of learning (Clemens et al., 2005, Clemens et al., 2006), but see also (Ackermann, Hartmann, Papassotiropoulos, de Quervain, & Rasch, 2015). However, sleep-dependent learning itself is correlated with intelligence (Fenn & Hambrick, 2015), and sleep spindle parameters show strong heritability (De Gennaro et al., 2008) as well as intra-individual stability (De Gennaro, Ferrara, Vecchio, Curcio, & Bertini, 2005; Finelli, Achermann, & Borbély, 2001). Thus, even the earliest of studies seem to have suggested that the correlation between sleep spindle parameters and learning may be moderated by trait-like cognitive abilities such as general intelligence (Bódizs et al., 2005; Nader & Smith, 2001), and that the plastic processes sleep spindles enable may support general cognitive ability outside the limited laboratory contexts involving learning and recall. Additionally, some studies (Bódizs, Gombos, Ujma, & Kovács, 2014; Ujma et al., 2014; Ujma, Sándor, Szakadát, Gombos, & Bódizs, 2016) have also found a sexually dimorphic pattern, with stronger associations in females.
General cognitive ability shows high heritability (Bouchard & McGue, 2003; Plomin & Deary, 2015) and a correlation with outcomes such as work success, income, physical and mental health (Calvin et al., 2017; Čukić, Brett, Calvin, Batty, & Deary, 2017; Gottfredson, 1997; Gottfredson & Deary, 2004; Lubinski, Benbow, & Kell, 2014; Schmidt & Hunter, 1998; Schmidt, Oh, & Shaffer, 2016) being some of the best-replicated findings in the history of psychology. While head and brain size (Pietschnig, Penke, Wicherts, Zeiler, & Voracek, 2015), chronometric variables (Jensen, 2006), neural efficiency defined as a reduced task-related activation (Neubauer & Fink, 2009) as well as both brain structure and connectivity mainly in the frontal and parietal lobes (Jung & Haier, 2007) are all associated with intelligence, its biological mechanisms are far from fully known. Therefore, the presence of an association between sleep spindle parameters and general cognitive ability may meaningfully add to the understanding of the biological underpinnings of the latter.
Several small size studies (median N = 24) testing the hypothesis of a spindle-IQ association were summarized in a recent study (Ujma, 2018) which found an association between IQ and sleep spindle amplitude, but not with other spindle parameters. However, a potential publication bias may result in unpublished negative or null findings (Cordi et al., 2014; Ferguson & Heene, 2012; Giner-Sorolla, 2012; Lilienfeld, 2017). Therefore, replications in large datasets are urgently needed. We set out to confirm the possibility of an association between sleep spindle parameters and cognitive ability in the Finnish birth cohort (N = 176), the largest sample currently available with full-night polysomnography data and intelligence scores. We investigated this relationship using two automated spindle detection methods, and hypothesized that the association between spindle parameters and intelligence is sexually dimorphic and more prominent in females (Ujma et al., 2014).
2. Methods
2.1. Participants
The participants came from the longitudinal Glaku (Glycyrrhizin in licorice) –study (Räikkönen et al., 2009, 2017; Strandberg, Järvenpää, Vanhanen, & McKeigue, 2001), building on a community cohort comprising individuals born healthy in Helsinki maternity hospital in 1998 (N initial = 1049). 451 (65% of the invited, 48% males) children participated in the previous, 12-year follow-up of this cohort in 2010–2011 (Pesonen et al., 2014). In years 2014–15, all the cohort members who had valid sleep data from the 12-year follow-up, and who lived within a 30 km radius from Helsinki (N = 279, 61.9% of the participants of the previous follow-up) were invited to a new follow-up. Of the invited, 197 (70.6%) participated at the age of 17 (Mean age = 16.9, SD = 0.1 years). The analytic sample with sleep and IQ data fully available comprised 176 adolescents (100 females and 76 males). They did not differ from the initial cohort (N = 1049) in terms of birthweight, gestational age, mother’s age and parents’ self-reported highest education (all P-values ≥.06). With regard to the previous follow-up at age 12 (N = 451), the current sample did not differ significantly in terms of IQ (Mean Difference 0.14 SD units, P = 0.09). The sample comprised 8 participants with diagnosed neurological or psychiatric disorders (learning difficulties (N = 3), depression (N = 3), panic disorder (N = 1) and ADHD (N = 1)). All subjects provided written informed consent, and the study was approved by the Ethical Committee in the University Central Hospital and conducted in line with the requirements laid down in the Declaration of Helsinki.
2.2. Cognitive abilities
Cognitive assessments were conducted at the homes of the participants in a quiet room, with a shortened version of the Wechsler Adult Intelligence Scale III (WAIS-III) (Wechsler, 1997). Four WAIS-III subtests were administered as instructed in the manual in the following order: Vocabulary, Block Design, Similarities, and Matrix Reasoning. The assessment started between 6 and 7 p.m. with a short questionnaire about possible factors affecting testing, e.g. handedness, native language and possible sensory or motor handicaps.
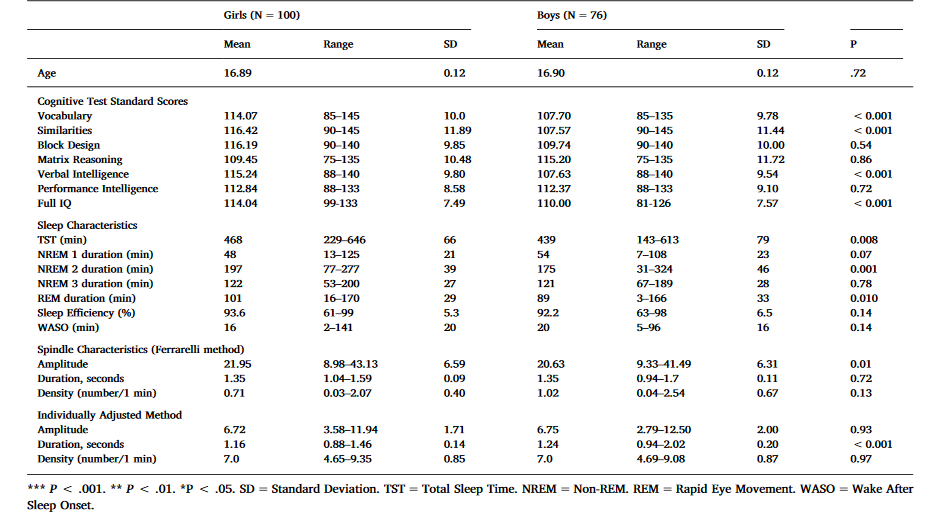
Verbal Intelligence (VIQ) was calculated by averaging the age-standardized Vocabulary and Similarities scores, and Perceptual Intelligence (PIQ) was calculated by averaging age-standardized Block Design and Matrix Reasoning scores. A full IQ estimate averaged the age-standardized scores of all four subtests. In this sample the age-standardized subtest scores (Table 1) seem elevated compared to the Finnish WAIS-III standards. However, the Finnish standardization has been recognized non-representative (Roivainen, 2013) with probable bias to heightened standard points. The VIQ and PIQ scores in this sample were normally distributed (data not shown).
Table 1. Sample characteristics – mean, range, standard deviation and the significance of gender difference.
2.3. Sleep EEG recording
Overnight polysomnographic sleep recording (PSG) was conducted in the homes of the participants with SOMNOscreen plus (SOMNOmedics GmbH, Germany). The sleep EEG electrodes were then attached and the recording was started. The subjects were instructed to follow their normal sleep routine. The next morning’s follow-up was scheduled according to the subjects’ timetable. Electroencephalography (EEG) measurements were recorded according to the guidelines of the American Association of Sleep Medicine with gold cup electrodes at 6 EEG locations (F3, F4, C3, C4, O1 and O2) and two channels for the mastoids (A1, A2) according to the standardized 10/20 system. The electro-oculogram (EOG) and the electromyogram (EMG) were measured by using disposable adhesive electrodes (Ambu Neuroline 715, Ambu A/S, Denmark), using two locations for EOG and three locations for EMG. In addition, an online reference Cz and a ground electrode in the middle of forehead were used. The sampling rate was 256 Hz. All signals were filtered with pass band of 0.5–40 Hz (Hamming windowed sinc zero-phase FIR filter, cut-off (-6 dB) 0.25 Hz and 44.3 Hz respectively) and re-referenced to the average signal of A1 and A2 electrodes. Sleep stages from PSG data were scored manually with the DOMINO program (v2.7; SOMNOmedics GmbH, Germany) by three experienced researchers in 30-s epochs based on standard criteria (Iber, Ancoli-Israel, Chesson, & Quan, 2007). Artifact rejection was based on visual inspection of the data during the manual staging.
2.4. Spindle analysis
We implemented two separate spindle detection methods due to the absence of a golden standard spindle detection methodology.
Method by Ferrarelli et al. Spindles were computationally extracted with the method described by Ferrarelli (Ferrarelli et al., 2010). The manually scored PSG signals were converted to EDF format in DOMINO software and then further analyzed for spindle detection by using functions of EEGlab 13.5.4b running on Matlab R2015a. We detected spindles in N2 sleep epochs where the impedance value was equal or lower than 10 kΩ during the corresponding 30-s epoch. The spindle analysis was conducted in three different frequency bands (10–16 Hz, 10–13 Hz, and 13–16 Hz) in order to differentiate between slow and fast spindles. Spindle detection threshold values for each channel were defined by the mean of the channel amplitude (μV) multiplied by 2 (lower limit) and 8 (higher limit) including all valid epochs (sleep stage N2 and impedance both at the target and reference electrodes ≤10 kΩ). Thus, we used channel-specific threshold definitions, taking into account that signal voltage may vary across the channels. Furthermore, only spindle events with a commonly used (Gruber & Wise, 2016; O’Reilly & Nielsen, 2015) duration of 500–2000 msec (counting from the time point with maximal amplitude) were considered for analysis. Signal amplitude was required to stay under the lower threshold for 78.1 ms which is approximately the duration of one sine period at 13 Hz. This was done in order to prevent false alarms in spindle detection.
We calculated the mean values of each subject for spindle amplitude (μV), duration (seconds), and density (number/min) from Stage 2 sleep. If the spindle number in a derivation was <5, no values were extracted from that derivation.
2.5. Individually adjusted method (IAM)
We replicated our analyses using an alternative sleep spindle detector, the Individual Adjustment Method (IAM) (Bódizs, Körmendi, Rigó, & Lázár, 2009). The IAM defines spindles as oscillations in the sigma (9–16 Hz) frequency range which are sufficiently large to contribute to a spectral peak. The IAM detects sleep spindles within individually defined slow and fast spindle frequency ranges and electrode-specific amplitude thresholds. The IAM first performs a high-resolution power spectral density (PSD) analysis, and defines slow and fast spindle spectral peaks based on the zero-crossings of the second-order derivative of the PSD as a function of frequency. Slow and fast spindle frequency ranges are thus defined based on spectral peaks. Next, this method filters the EEG signal to the individually defined slow and fast spindle frequency ranges, and detects spindles wherever the amplitude of this signal is sufficiently large to contribute to a spectral peak on that electrode.
2.6. Covariates
Maternal licorice consumption during pregnancy, which has previously been associated with cognitive abilities of the offspring in this cohort (Räikkönen et al., 2009; Räikkönen et al., 2017), was derived from the maternal report after the birth and categorized as low, moderate or high usage.
2.7. Statistical analysis
To avoid multiple testing and to reduce Type I error, linear mixed models with a random intercept were used to examine the associations between cognitive test scores and spindle amplitude, duration, density among all participants and separately in both sexes. This was because many previous findings indicated the presence of a sex difference (Ujma et al., 2014). All analyses were run separately for slow and fast spindles. In case of statistically significant main effects, we tested the significance of two-way interactions ‘EEG derivation (central, frontal) *IQ’ and ‘IQ *sex’ in predicting spindle characteristics. All analyses were repeated after excluding 8 participants with a neurological or psychiatric condition.
We ran two models, first with unadjusted associations, in the second model adjusting for sex (only in the pooled group), and maternal prenatal licorice consumption. As a replication, we rerun the analyses with the alternative IAM spindle detection method. We tested associations between spindle activity and cognitive ability score controlling for Type I error rate due to multiple testing with a false detection rate procedure (FDR) (Benjamini & Hochberg, 1995) setting the false discovery rate across 2 × 12 tests at 0.05 for IAM and Ferrarelli methods. VIQ and PIQ were highly correlated with Full IQ (r > 0.78 P < 0.001).
3. Results
3.1. Initial analyses
As shown in Table 1, girls had significantly higher verbal intelligence scores than boys. Girls had also 29 min longer TST, 22 min longer NREM, and 12 min longer REM sleep than boys. Regarding spindle characteristics, girls had significantly higher spindle amplitudes than boys in the Ferrarelli method and with the IAM, boys had longer duration of spindles than girls.
3.2. Spindles and cognitive ability at age 17
3.2.1. Slow spindles, Ferrarelli method
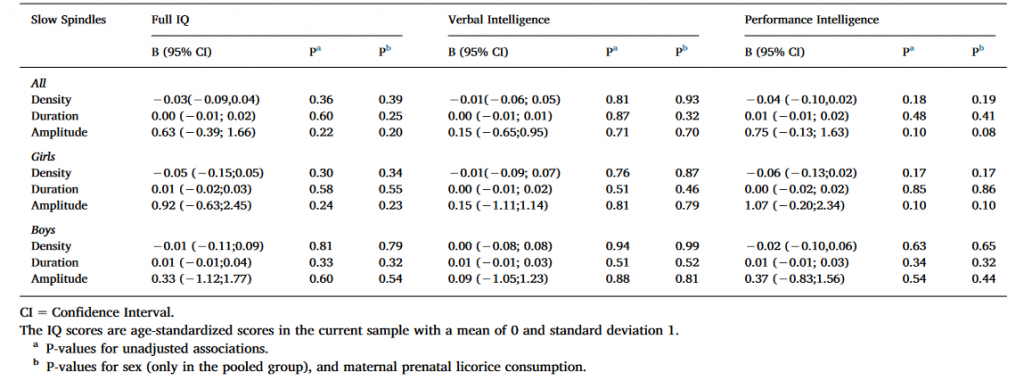
As Table 2 shows, the associations between IQ measures and spindle characteristics were non-significant for all subjects as well as girls and boys analyzed separately in both the non-adjusted model and in the models adjusted for sex, and maternal prenatal licorice consumption.
Table 2. Linear mixed model analyses between cognitive reasoning and slow spindle characteristics with Ferrarelli spindle detection method.
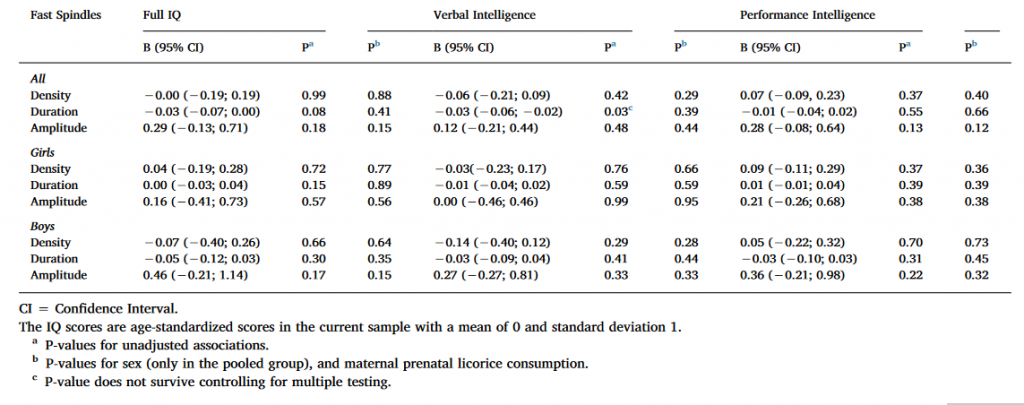
3.2.2. Fast spindles, Ferrarelli method
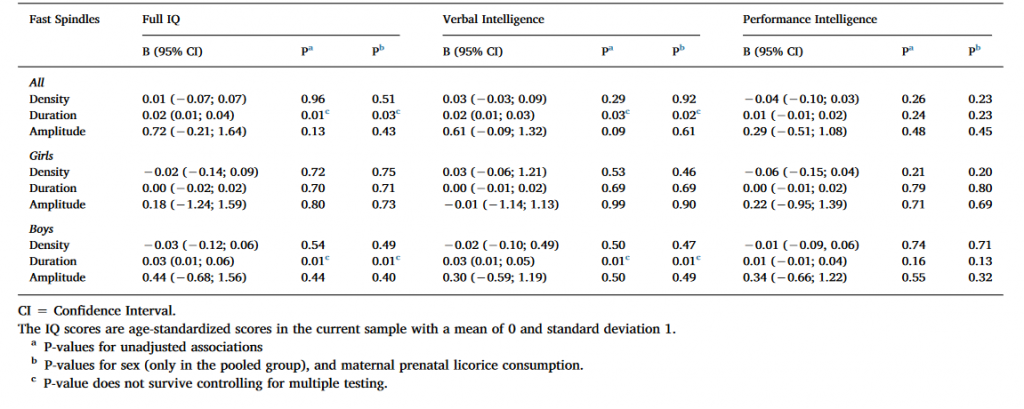
As Table 3 shows, Full IQ and VIQ were positively associated with spindle duration in the pooled sample and among boys in the unadjusted models using uncorrected significance levels (P < 0.05). The nominally significant results regarding spindle duration remained significant also in the second adjusted model. However, none of these associations remained significant after FDR corrections. None of the significant main effects were moderated by the electrode location or sex (for all interaction effects P > 0.12 and > 0.08, respectively).
Table 3. Linear mixed model analyses between cognitive reasoning and fast spindle characteristics with Ferrarelli spindle detection.
3.2.3. Slow spindles, IAM method
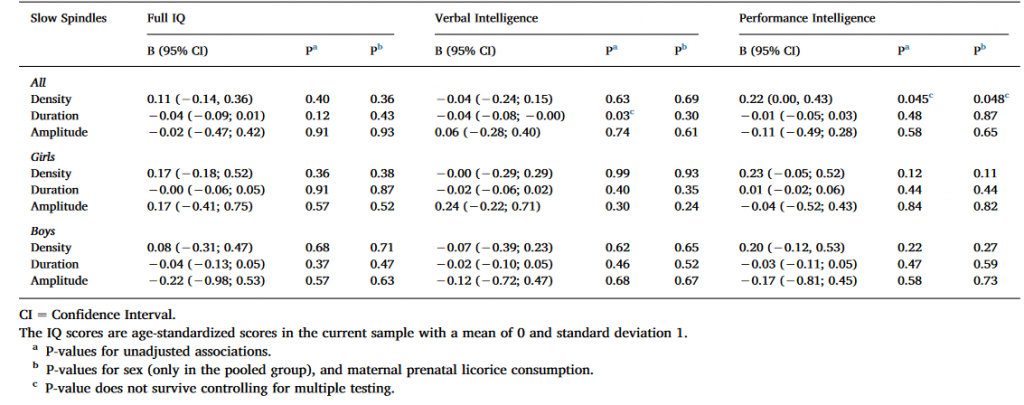
As indicated in Table 4, two nominally significant associations emerged: one between PIQ and spindle density in both models, and another between VIQ and spindle duration in the unadjusted model, both in the full sample. None of these associations remained significant after FDR corrections.
Table 4. Linear mixed model analyses between cognitive reasoning and slow spindle characteristics with IAM spindle detection methods.
3.2.4. Fast spindles, IAM method
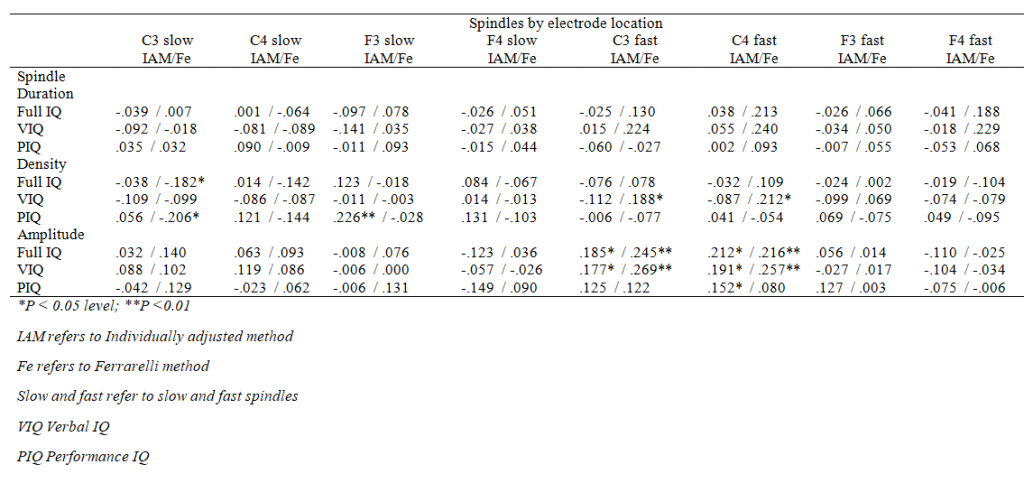
As indicated in Table 5, only one nominally significant association emerged between spindle duration and verbal IQ in the unadjusted model of the full sample that did not survive FDR corrections. None of the significant main effects were moderated by the electrode location or sex (for all interaction effects P > 0.09 and > 0.06, respectively). Supplementary Tables 1 and 2 show all the associations by electrodes.
Table 5. Linear mixed model analyses between cognitive reasoning and fast spindle characteristics with IAM spindle detection methods.
3.2.5. Exclusion of participants with neurologic or psychiatric conditions
We rerun all the crude models after excluding the 8 participants who self-reported to have diagnosis of a neurologic or psychiatric condition. This exclusion did not affect any of the non-significant or significant associations.
4. Discussion
We studied the relationship between cognitive ability (measured in late adolescence) and sleep spindle characteristics among 176 adolescents belonging to a community-based cohort. This represents the largest study on the association between IQ measures and sleep spindles, and the only study using two different spindle detection methods.
Against the initial assumptions, we did not find significant associations between sleep spindles and IQ measures with either spindle detection method, even though we used a conservative method to correct Type I error, not counting the two spindle detection methods and the several IQ subtests as separate comparisons. Even using this mild correction, only one significant association emerged, between Full IQ and fast spindle duration calculated using the Ferrarelli method (B = 0.02, 95% CI 0.01–0.04, P = 0.01). Even this effect was not replicated by the IAM method (B = −0.03, 95% CI −0.07−0.00, P = 0.08). No effects were moderated by sex or electrode location, providing no evidence that the association between spindle measures and intelligence is sexually dimorphic. Excluding individuals with either a neurologic or psychiatric condition did not affect the findings.
Our results are at odds with much of the previous literature about a general positive relationship between sleep spindle measures and intelligence. However, the small sample size of most studies means that substantial sampling error is expected around the true population correlation, resulting in a large number of studies reporting false positive effects using significance level-based null hypothesis testing methods. In addition to sampling error, spindle data is usually rich in different parameters, leaving an opportunity to do multiple testing without either reporting it or publishing the results without appropriate statistical correction for Type I error. On the other hand, small sample sizes may equally lead to lack of statistical power to detect small effects.
While neither spindle detection method yielded substantial correlations between spindle measures and intelligence, the methods have different approaches to spindle detection. Both methods adjust individually the spindle detection thresholds, the Ferrarelli method regarding amplitude and IAM for frequency. In the Ferrarelli method slow and fast spindle frequency is pre-defined, while in the IAM method, it is determined empirically based on the visible spectral peaks of the scalp EEG signal. Empirically determined spindle frequencies may not always overlap with pre-defined (11–13 Hz for slow spindles and 13–15 Hz for fast spindles) ranges (Ujma et al., 2015). Even with controlling for this potential frequency bias with the IAM method, there were no significant associations, confirming the validity of our findings.
The only initial associations we found were between IQ and spindle duration and they did not survive FDR correction. It is notable that overall pattern of associations were parallel (showing null effects) with both the detectors. In sum, in the largest available single study of the association between sleep spindle parameters and cognitive ability we were unable to replicate previous positive findings, including a significant sex interaction.
5. Strengths and limitations
The strength of the present study was the large, community-based longitudinal sample, with a high age coherence and a substantial sample of both girls and boys, while using two automatic spindle detection methods in the same study increased the reliability of the study. Notably, there is currently no golden standard or consensus regarding automated spindle detection methods. They differ for instance in terms of spindle duration definition, conventionally being around 500–2000 msec, as in our Ferrarelli-based method. In addition, some detectors adjust for the individual amplitude of the sleep EEG (e.g. Ferrarelli) and some not. As Table 1 showed, this results in different amplitude levels also across the methods used in this study. With regard to frequency, the 10–16 Hz is a general, and widely-used spindle frequency range (Hagler et al., 2018; Huupponen et al., 2000; McClain et al., 2016; Nir et al., 2011). Its separation into slow and fast subranges with somewhat variable frequencies is also a convention in sleep EEG research (De Gennaro & Ferrara, 2003; Fogel & Smith, 2011). However, the division to slow and fast spindles may differ across methods. For example, our Ferrarelli-based method uses the conventional 10–13 Hz slow and 13–16 Hz fast spindle ranges, and IAM uses empirically determined frequencies based on EEG spectral peaks.
Our study has also some limitations. The academic family backgrounds of the participants were biased towards higher educational status. Even though the statistical difference was not significant when comparing to the earlier follow-up of the research project, greater attrition in families of low education was already reported at that time (Pesonen et al., 2014). However, in relation to the latest follow-up at age 12, we did not find IQ-related attrition in the current study. It does not exclude the potential Type II error though, assuming that the IQ range in this sample is more restricted than among the entire population. Having only one night with PSG recording can also be considered a limitation. However, the wake-after-sleep-onset minutes in our study were very comparable to age norms, percentage of N1 sleep being only slightly higher (Ohayon, Carskadon, Guilleminault, & Vitiello, 2004). Also, EEG spectral components in the spindle range (De Gennaro et al., 2005; De Gennaro et al., 2008) but also sleep spindle parameters (Reynolds, Short, & Gradisar, 2018), show high inter-night reliabilities, meaning that it is unlikely that using first-night effects introduced further Type II error. It is also probable that a two-night procedure (one night for habituating, another for measurement) would have lowered willingness to participate, resulting in a smaller and much less representative sample. Although the home environment provided a natural and stress-free sleeping environment, and the testing proceeded strictly according the preset assessment protocol, there was some variation in the time of assessment in relation to individual’s bedtime. In addition, our study was conducted in adolescents at an age when a dynamic development of the brain is still taking place. Further studies are needed to clarify whether our findings can be generalized to other age ranges. Finally, there is currently no golden standard or consensus regarding automated spindle detection methods. The convergence between different methods requires further investigation.
6. Conclusions
This study, using the largest study cohort currently available, failed to find substantial associations between general cognitive ability and spindle properties in both sexes. The sample size covering this topic is the largest thus far reported, indicating no association between sleep spindles and IQ. This was replicated across the different sleep spindle detection methods. Previous studies have been limited in size, and their findings probably overestimated the strength of the true association between spindle parameters and cognitive ability. Based on our study, we conclude that sleep spindle parameters are not substantially associated with general intelligence at a level detectable with this sample size with adequate power, and the previously reported sex differences in this association could not be replicated in this sample.
Conflict of interest
The authors declare no conflict of interest.
Funding Source
The study was supported by the Academy of Finland, and the Ane och Signe Gyllenberg Foundation.
Appendix A. Supplementary data
Supplementary Table 1. Correlation coefficients between IQ measures and spindle activity, all participants together
References
Ackermann, S., Hartmann, F., Papassotiropoulos, A., de Quervain, D. J.-F., & Rasch, B. (2015). No associations between interindividual differences in sleep parameters and episodic memory consolidation. Sleep, 38(6), 951–959. https://doi.org/10.5665/sleep.4748.
Benjamini, Y., & Hochberg, Y. (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B (Methodological). https://doi.org/10.2307/2346101 WileyRoyal Statistical Society.
Bódizs, R., Gombos, F., Ujma, P. P., & Kovács, I. (2014). Sleep spindling and fluid intelligence across adolescent development: Sex matters. Frontiers in Human Neuroscience, 8(952), https://doi.org/10.3389/fnhum.2014.00952.
Bódizs, R., Kis, T., Lazar, A. S., Havran, L., Rigo, P., Clemens, Z., & Halasz, P. (2005). Prediction of general mental ability based on neural oscillation measures of sleep. Journal of Sleep Research, 14(3), 285–292. https://doi.org/10.1111/j.1365-2869.2005.00472.x.
Bódizs, R., Körmendi, J., Rigó, P., & Lázár, A. S. (2009). The individual adjustment method of sleep spindle analysis: Methodological improvements and roots in the fingerprint paradigm. Journal of Neuroscience Methods, 178(1), 205–213. https://doi.org/10.1016/j.jneumeth.2008.11.006.
Bouchard, T. J., & McGue, M. (2003). Genetic and environmental influences on human psychological differences. Journal of Neurobiology, 54(1), 4–45. https://doi.org/10.1002/neu.10160.
Calvin, C. M., Batty, G. D., Der, G., Brett, C. E., Taylor, A., Pattie, A., & Deary, I. J. (2017). Childhood intelligence in relation to major causes of death in 68 year follow-up: Prospective population study. BMJ (Clinical Research Ed.), 357, j2708.https://doi.org/10.1136/BMJ.J2708.
Clemens, Z., Fabó, D., & Halász, P. (2005). Overnight verbal memory retention correlates with the number of sleep spindles. Neuroscience, 132(2), 529–535. https://doi.org/10.1016/j.neuroscience.2005.01.011.
Clemens, Z., Fabó, D., & Halász, P. (2006). Twenty-four hours retention of visuospatial memory correlates with the number of parietal sleep spindles. Neuroscience Letters, 403(1–2), 52–56. https://doi.org/10.1016/j.neulet.2006.04.035.
Cordi, M., Ackermann, S., Bes, F. W., Hartmann, F., Konrad, B. N., Genzel, L., … Dresler, M. (2014). Lunar cycle effects on sleep and the file drawer problem. Current Biology,24(12), R549–R550. https://doi.org/10.1016/J.CUB.2014.05.017.
Čukić,I., Brett, C. E., Calvin, C. M., Batty, G. D., & Deary, I. J. (2017). Childhood IQ and survival to 79: Follow-up of 94% of the Scottish Mental Survey 1947. Intelligence, 63,45–50. https://doi.org/10.1016/j.intell.2017.05.002.
De Gennaro, L., & Ferrara, M. (2003). Sleep spindles: An overview. Sleep Medicine Reviews,7(5), 423–440. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/14573378.
De Gennaro, L., Ferrara, M., Vecchio, F., Curcio, G., & Bertini, M. (2005). An electro-encephalographic fingerprint of human sleep. NeuroImage, 26(1), 114–122. https://doi.org/10.1016/j.neuroimage.2005.01.020.
De Gennaro, L., Marzano, C., Fratello, F., Moroni, F., Pellicciari, M. C., Ferlazzo, F., Rossini, P. M. (2008). The electroencephalographic fingerprint of sleep is genetically determined: A twin study. Annals of Neurology, 64(4), 455–460. https://doi.org/10.1002/ana.21434.
Fenn, K. M., & Hambrick, D. Z. (2015). General intelligence predicts memory change across sleep. Psychonomic Bulletin & Review, 22(3), 791–799. https://doi.org/10.3758/s13423-014-0731-1.
Ferguson, C. J., & Heene, M. (2012). A Vast Graveyard of undead theories. Perspectives on Psychological Science, 7(6), 555–561. https://doi.org/10.1177/1745691612459059.
Ferrarelli, F., Peterson, M. J., Sarasso, S., Riedner, B. A., Murphy, M. J., Benca, R. M., Tononi, G. (2010). Thalamic dysfunction in schizophrenia suggested by whole-night deficits in slow and fast spindles. American Journal of Psychiatry, 167(11), 1339–1348. https://doi.org/10.1176/appi.ajp.2010.09121731.
Finelli, L., Achermann, P., & Borbély, A. A. (2001). Individual “Fingerprints” in human sleep EEG topography. Neuropsychopharmacology, 25(5), S57–S62. https://doi.org/10.1016/S0893-133X(01)00320-7.
Fogel, S. M., & Smith, C. T. (2011). The function of the sleep spindle: A physiological index of intelligence and a mechanism for sleep-dependent memory consolidation. Neuroscience & Biobehavioral Reviews, 35(5), 1154–1165. https://doi.org/10.1016/j.neubiorev.2010.12.003.
Giner-Sorolla, R. (2012). Science or art? How aesthetic standards grease the way through the publication bottleneck but undermine science. Perspectives on Psychological Science, 7(6), 62–571. https://doi.org/10.1177/1745691612457576.
Gottfredson, L. S. (1997). Why g matters: The complexity of everyday life. Intelligence, 24(1), 79–132. https://doi.org/10.1016/S0160-2896(97)90014-3.
Gottfredson, L. S., & Deary, I. J. (2004). Intelligence predicts health and longevity, but why? Current Directions in Psychological Science, 13(1), 1–4. https://doi.org/10.1111/j.0963-7214.2004.01301001.x.
Gruber, R., & Wise, M. S. (2016). Sleep spindle characteristics in children with neuro-developmental disorders and their relation to cognition. Neural Plasticity, 2016(4724792), https://doi.org/10.1155/2016/4724792.
Hagler, D. J., Ulbert, I., Wittner, L., Erőss, L., Madsen, J. R., Devinsky, O., & Halgren, E. (2018). Heterogeneous origins of human sleep spindles in different cortical layers. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 38(12),3013–3025. https://doi.org/10.1523/JNEUROSCI.2241-17.2018.
Huupponen, E., Varri, A., Himanen, S.-L., Hasan, J., Lehtokangas, M., & Saarinen, J. (2000). Optimization of sigma amplitude threshold in sleep spindle detection. Journal of Sleep Research, 9(4), 327–334. https://doi.org/10.1046/j.1365-2869.2000.00220.x.
Iber, C., Ancoli-Israel, S., Chesson, A. L., & Quan, S. (2007). The AASM manual for the scoring of sleep and associated events: Rules, terminology and technical specifications. Westchester, IL: American Academy of Sleep Medicine.
Jensen, A. R. (2006). Clocking the mind: Mental chronometry and individual differences. Elsevier.
Jung, R. E., & Haier, R. J. (2007). The Parieto-Frontal Integration Theory (P-FIT) of intelligence: Converging neuroimaging evidence. Behavioral and Brain Sciences, 30(02),135. https://doi.org/10.1017/S0140525X07001185.
Lilienfeld, S. O. (2017). Psychology’s replication crisis and the grant culture: Righting the ship. Perspectives on Psychological Science, 12(4), 660–664. https://doi.org/10.1177/1745691616687745.
Lubinski, D., Benbow, C. P., & Kell, H. J. (2014). Life paths and accomplishments of mathematically precocious males and females four decades later. Psychological Science, 25(12), 2217–2232. https://doi.org/10.1177/0956797614551371.
Lüthi, A. (2014). Sleep spindles. The Neuroscientist, 20(3), 243–256. https://doi.org/10.1177/1073858413500854.
McClain, I. J., Lustenberger, C., Achermann, P., Lassonde, J. M., Kurth, S., & LeBourgeois, M. K. (2016). Developmental changes in sleep spindle characteristics and sigma power across early childhood. Neural Plasticity, 2016,1–9. https://doi.org/10.1155/2016/3670951.
Nader, R. S., & Smith, C. (2001). The relationship between stage 2 sleep spindles and intelligence. Sleep, 24, A160.
Neubauer, A. C., & Fink, A. (2009). Intelligence and neural efficiency: Measures of brain activation versus measures of functional connectivity in the brain. Intelligence, 37(2),223–229. https://doi.org/10.1016/J.INTELL.2008.10.008.
Nir, Y., Staba, R. J., Andrillon, T., Vyazovskiy, V. V., Cirelli, C., Fried, I., & Tononi, G. (2011). Regional slow waves and spindles in human sleep. Neuron, 70(1), 153–169. https://doi.org/10.1016/j.neuron.2011.02.043.
Ohayon, M. M., Carskadon, M. A., Guilleminault, C., & Vitiello, M. V. (2004). Meta-analysis of quantitative sleep parameters from childhood to old age in healthy in-dividuals: Developing normative sleep values across the human lifespan. Sleep, 27(7),1255–1273. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/15586779.
O’Reilly, C., & Nielsen, T. (2015). Automatic sleep spindle detection: Benchmarking with fine temporal resolution using open science tools.Frontiers in Human Neuroscience, 9(353), https://doi.org/10.3389/fnhum.2015.00353.
Pesonen, A.-K., Martikainen, S., Heinonen, K., Wehkalampi, K., Lahti, J., Kajantie, E., & Räikkönen, K. (2014). Continuity and change in poor sleep from childhood to early adolescence. Sleep, 37(2), 289–297. https://doi.org/10.5665/sleep.3400.
Pietschnig, J., Penke, L., Wicherts, J. M., Zeiler, M., & Voracek, M. (2015). Meta-analysis of associations between human brain volume and intelligence differences: How strong are they and what do they mean? Neuroscience & Biobehavioral Reviews, 57,411–432. https://doi.org/10.1016/j.neubiorev.2015.09.017.
Plomin, R., & Deary, I. J. (2015). Genetics and intelligence differences: Five special findings. Molecular Psychiatry, 20(1), 98–108. https://doi.org/10.1038/mp.2014.105.
Räikkönen, K., Martikainen, S., Pesonen, A.-K., Lahti, J., Heinonen, K., Pyhälä, R., Kajantie, E. (2017). Maternal licorice consumption during pregnancy and pubertal, cognitive, and psychiatric outcomes in children. American Journal of Epidemiology, 185(5), 317–328. https://doi.org/10.1093/aje/kww172.
Räikkönen, K., Pesonen, A.-K., Heinonen, K., Lahti, J., Komsi, N., Eriksson, J. G., Strandberg, T. E. (2009). Maternal licorice consumption and detrimental cognitive and psychiatric outcomes in children. American Journal of Epidemiology, 170(9),1137–1146. https://doi.org/10.1093/aje/kwp272.
Rasch, B., & Born, J. (2013). About sleep’s role in memory. Physiological Reviews, 93(2),681–766. https://doi.org/10.1152/physrev.00032.2012.
Reynolds, C. M., Short, M. A., & Gradisar, M. (2018). Sleep spindles and cognitive performance across adolescence: A meta-analytic review. Journal of Adolescence, 66,55–70. https://doi.org/10.1016/j.adolescence.2018.04.003.
Roivainen, E. (2013). Are cross-national differences in iq profiles stable? A comparison of Finnish and U.S. WAIS norms. International Journal of Testing, 13(2), 140–151. https://doi.org/10.1080/15305058.2012.657315.
Rosanova, M., & Ulrich, D. (2005). Pattern-specific associative long-term potentiation induced by a sleep spindle-related spike train. Journal of Neuroscience, 25(41),9398–9405. https://doi.org/10.1523/JNEUROSCI.2149-05.2005.
Schmidt, F. L., & Hunter, J. E. (1998). The validity and utility of selection methods in personnel psychology: Practical and theoretical implications of 85 years of research findings. Psychological Bulletin, 124(2), 262–274. https://doi.org/10.1037/0033-2909.124.2.262.
Schmidt, F. L., Oh, I., & Shaffer, J. A. (2016, October 17). The validity and utility of selection methods in personnel psychology: Practical and theoretical implications of 100 years of research findings. Retrieved from https://papers.ssrn.com/sol3/papers.cfm?abstract_id=2853669.
Steriade, M. (2003). The corticothalamic system in sleep. Frontiers in Bioscience: A Journal and Virtual Library, 8, d878–d899. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12700074.
Strandberg, T. E., Järvenpää, A. L., Vanhanen, H., & McKeigue, P. M. (2001). Birth outcome in relation to licorice consumption during pregnancy. American Journal of Epidemiology, 153(11), 1085–1088. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11390327.
Ujma, P. P. (2018). Sleep spindles and general cognitive ability – A meta-analysis. Sleep Spindles & Cortical Up States, 1–17. https://doi.org/10.1556/2053.2.2018.01.
Ujma, P. P., Bódizs, R., Gombos, F., Stintzing, J., Konrad, B. N., Genzel, L., & Dresler, M. (2015). Nap sleep spindle correlates of intelligence. Scientific Reports, 5(1), 17159. https://doi.org/10.1038/srep17159.
Ujma, P. P., Konrad, B. N., Genzel, L., Bleifuss, A., Simor, P., Pótári, A., … Dresler, M. (2014). Sleep spindles and intelligence: Evidence for a sexual dimorphism. The Journal of Neuroscience, 34(49), 16358–16368. https://doi.org/10.1523/JNEUROSCI.1857-14.2014.
Ujma, P. P., Sándor, P., Szakadát, S., Gombos, F., & Bódizs, R. (2016). Sleep spindles and intelligence in early childhood–developmental and trait-dependent aspects. Developmental Psychology, 52(12), 2118–2129. https://doi.org/10.1037/dev0000233.
Urakami, Y., Ioannides, A. A., & Kostopoulos, G. K. (2012). Sleep spindles – As a bio-marker of brain function and plasticity. Advances in Clinical Neurophysiology (pp. 73–108). InTech. https://doi.org/10.5772/48427.
Wechsler, D. (1997). WAIS-III administration and scoring manual. San Antonio, TX: The Psychological Corporation.
© 2018 Elsevier Inc. All rights reserved.