Dreaming Vol 26(1) 58-78. link
DOI: 10.1037/drm0000022
Piroska Sándor
Institute of Behavioural Sciences, Semmelweis University Budapest, Hungary
Center for Child and Family Welfare, Child Psychology Service, Vecsés, Hungary
Sára Szakadát
Institute of Behavioural Sciences, Semmelweis University Budapest, Hungary
Róbert Bódizs
Institute of Behavioural Sciences, Semmelweis University Budapest, Hungary
Department of General Psychology, Pázmány Péter Catholic University, Budapest, Hungary
Abstract
The development of dreaming and its association with brain maturation and cognitive development are rarely studied in spite of adult studies showing a close relationship between dreaming and cognitive functioning. In order to bridge this gap in the literature we aimed to depict the associations between individual differences in neurocognitive maturation and the formal and content related characteristics of children’s dream reports. We analysed the dream reports of 40 children between the ages of 4 to 8 years. Specific dream content categories, found to change significantly throughout development, were correlated with cognitive performance. To measure the latter we used neuropsychological tests (a modified version of the Fruit Stroop Test and Emotional Stroop Test for Children, and the Attention Network Test (ANT)) and intelligence tests (subscales of the Wechsler Intelligence Scale for Children (WISC), and the RAVEN Coloured Progressive Matrices (CPM)). Results suggest that the dreamer’s presence in their dreams (manifested in activities, interactions, self-effectiveness, wilful effort and cognitive reflections) indicates more effective executive control in waking life. The quality and content of these activities and interactions are correlated with the child’s capacities of emotional processing. Contrary to previous findings dream bizarreness and dream recall frequency were not associated with any cognitive indicators. While in adult dream research the continuity of waking and dreaming cognition has been well-studied, our work is one of the first to explore the connection between children’s cognitive maturation and dreaming.
Keywords: dreams, child, cognitive development, maturation, intelligence, executive functions
The nature of dreaming in early childhood remains a matter of debate due to scant research and methodological challenges in dream report collection, and the lack of proof that dreaming is associated with REM sleep under a certain age (For a review see: Sándor, Szakadát, & Bódizs, 2014). REM sleep (that is associated with the most vivid and story-like dreaming in adults) appears at an early stage of foetal development and plays an important role in neural maturation in childhood (Dang-Vu, Desseilles, Peigneux, & Maquet, 2006; Mirmiran, 1995; Jenni & Dahl, 2008). This role inspired some authors to assume that dreaming is also present from a very young age and plays a crucial role in neural and cognitive development (Staunton, 2001). Others concluded, based on empirical investigation of children’s dreams, that dreaming is a purely cognitive achievement dependent on the maturation of visuospatial brain areas and therefore it only appears at around 2 years of age (Foulkes, 1982, 1999). Although we still do not know if dreaming is already associated with REM sleep in infancy or if it is accomplished later on the basis of cognitive and emotional development, it is evident from existing research that dreaming and dream narratives in children develop parallelly to some cognitive, intellectual and social abilities (Colace, 2010; Foulkes, 1982, 1999).
In a series of systematic longitudinal and cross-sectional studies of children’s dreams, using laboratory awakenings for data collection, they found the Block Design test from the Wechsler Intelligence Scale for Children (WISC) to be a reliable and stable correlate of dream report frequency for 5 to 15 year-old children (Foulkes, Hollifield, Sullivan, Bradley, & Terry, 1990; Foulkes, 1982, 1999). Surprisingly, neither working memory tests nor verbal skills reliably predicted the rate of dream recall of these children: the strong correlation between dream recall frequency and the development of visuospatial skills combined with the absence of reliable correlation with memory and verbal skills motivated Foulkes to believe that dream recall frequency is more dependent on the ability to create dream imagery in the brain and does not reflect the children’s abilities to remember or communicate dreams (Foulkes, 1982, 1999). Interestingly, in Foulkes’ longitudinal study the only age group that did not fit the above pattern is the youngest age-group (3-to 5-year-olds), where dream report rate was correlated rather with social and verbal skills and not visuospatial abilities (Foulkes, 1982, 1999). It is important to note that this is also the age group for whom dream characteristics and content collected in different settings are the most divergent. While the aforementioned laboratory studies found the dream reports of preschoolers strikingly mundane (lacking movements, actions, an active self-character, human characters, interactions and feelings), a number of other studies, using home or kindergarten settings and morning dream interviews, showed children’s dream narratives as being much more similar to those of adults (emotional, eventful, most characters human, frequent interactions, representation of an active self) (Despert, 1949; Honig & Nealis, 2012; Resnick, Stickgold, Rittenhouse, & Hobson, 1994; Sándor, Szakadát, Kertész, & Bódizs, 2015).
The significant divergence in the research outcomes is most likely to be the result of different settings and dream collection methods, which question has long been the topic of passionate debates amongst the dream researchers. Some authors found home dreams in adults to be more dramatic than laboratory dreams (G W Domhoff & Kamiya, 1964; Hall & Van de Castle, 1966; Weisz & Foulkes, 1970) which was confirmed in adolescents (Strauch, 2004), but is still controversial in children (Foulkes, 1979). The most common critiques of the laboratory dream interviews are that the unknown environment and interviewer may cause disorientation or difficulties for the children to talk about their dreams (Bulkeley, Broughton, Sanchez, & Stiller, 2005; Resnick, Stickgold, Rittenhouse, & Hobson, 1994) or they may even influence the dream experience itself (Domhoff, 1969). In our opinion the method of nighttime awakenings might be unsuitable for preschoolers, who might not be able to fully arouse during the interviews, thus subjecting the dream report to be biased by their drowsy state of mind somewhere in between sleeping and wakefulness (for more details about the debate: Sándor, Szakadát, Kertész, & Bódizs, 2015, and for an example of nighttime dream report: Foulkes & Shepherd, 1971).
Other than the laboratory studies, which found reliable association between visuospatial skills and dream recall frequency (Foulkes et al., 1990; Foulkes, 1982, 1999), there are no systematic studies investigating the relationship between cognitive abilities and dream reports. Although direct correlations with cognitive measures were not presented, these studies reported an age-related increase in certain features of dream reports, such as motion imagery, active self-representation, representation of human characters, interactions, and voluntary actions (Foulkes, 1982, 1999; Strauch & Meier, 1996; Strauch, 2005). Some non-laboratory studies also found a similar age-related increase (Honig & Nealis, 2012; Oberst, Charles, & Chamarro, 2005; Sándor et al., 2015), which implies a parallel maturation of dream content with cognitive skills. Other than the above mentioned studies only isolated pieces of evidence can be found that show possible parallel development between characteristics of dream reports and cognitive maturation. Colace (2010), using home and kindergarten interviews, found a correlation between dream bizarreness and various cognitive abilities, such as linguistic skills, long term memory capacity, attention span, symbolization, visuospatial skills and superego development. Thus dream bizarreness seems to be the only dream content feature that has been shown to be correlated with cognitive processes and skills, not only in developmental but in adult studies as well (Cicogna, Occhionero, Natale, & Esposito, 2007; Colace, 2003).
Besides the behavioural correlations other measures that could enlighten us about the associations between cognitive development and dreaming would be studying the overlap between their neuro-anatomical correlates. The literature, based on brain lesion studies (Solms, 1997, 2003) and brain imaging techniques (Braun, 1997; Maquet et al., 1996), provides concordant information about the brain areas that are likely to be involved in dream formation. It has been discovered that the medial prefrontal cortex, anterior cingulate cortex, limbic region and basal forebrain together with the occipito-temporal region and visual association cortex are more active in REM sleep than in NREM sleep. These areas were also identified as part of the dreaming network by Solms’ lesion studies (Domhoff, 2001; Domhoff, 2011; Muzur, Pace-Schott, & Hobson, 2002; Nofzinger & Maquet, 2011). Moreover, during wakeful functioning the limbic structures such as amygdale are associated with emotional responses especially fear responses (Adolphs, Tranel, Damasio, & Damasio, 1995; Feinstein, Adolphs, Damasio, & Tranel, 2011), and the ventromedial prefrontal cortex is involved in emotion regulation and fear extinction processes (Hänsel & von Känel, 2008; Urry et al., 2006). These results support the psychological models that connect dreaming with one’s affective experiences and suppose an emotional regulational function of dreams (Cartwright, Luten, Young, Mercer, & Bears, 1998; Cartwright, 2011; Nielsen & Levin, 2007). Theories and empirical findings suggest that REM-sleep and/or dreaming may indeed promote the resolution of emotional difficulties and the improvement of next-day mood (Cartwright, Agargun, Kirkby, & Friedman, 2006; van der Helm et al., 2011; Walker & van der Helm, 2009). Importantly, specific dream content and characteristics have also been associated with the functioning of the above mentioned brain areas. For instance according to Nielsen and Levin’s neurocognitive theory, emotionally loaded dreams (especially nightmares) can be a consequence of a disruption in the cooperation of the emotionally loaded subcortical areas and prefrontal-cortical areas, which are unable to down-regulate those emotions, resulting in ineffective emotional regulation (Levin & Nielsen, 2007; Nielsen & Levin, 2007).
Interestingly, there are examples of frontal cortical areas, that are core supporters of executive attention, being also active during REM sleep (e.g.: anterior cingulate and orbitofrontal cortices, Nofzinger, Mintun, Wiseman, Kupfer, & Moore, 1997), although other areas of the executive functioning show decreased activation during REM sleep (e.g.: dorsolateral prefrontal cortex, Muzur, Pace-Schott, & Hobson, 2002). Common measures of executive attention are the Stroop Test (van Veen & Carter, 2005) and the Attention Network Test (ANT, J Fan & Posner, 2004). In a wider sense attention can be modelled as the interaction among three well defined, independent components – namely alerting, orienting and executive control (as appears in the ANT; Jin Fan et al., 2002) – with different neuroanatomical bases (Maurizio Corbetta & Shulman, 2002; Jin Fan, Flombaum, McCandliss, Thomas, & Posner, 2003). On the basis of previous studies on vigilance the alerting system (responsible for achieving and maintaining an alert state) was associated with right frontal and parietal regions (Coull, Frith, Frackowiak, & Grasby, 1996). The orienting network (responsible for the selection of information from sensory input) is associated with parietal and frontal areas, especially the superior parietal lobe and the temporo-parietal junction (Corbetta, Kincade, Ollinger, McAvoy, & Shulman, 2000). The executive system (measured by the Conflict Network in ANT and responsible for resolving conflict amongst responses) is often studied using conflict tests (such as the Stroop Test) which activate the middle frontal areas (anterior cingulate) and the lateral prefrontal cortex (MacDonald, 2000).
Nightmare sufferers have been found to exhibit impaired measures of waking frontal executive functions. They showed longer reaction times in the Go/NoGo Test when emotional distractors were presented and also showed a general slowing tendency in the emotional Stroop Test compared to healthy subjects (Simor, Pajkossy, Horváth, & Bódizs, 2012). At the other end of the dreaming continuum, lucidity (awareness of dreaming during the dream itself) was correlated positively with the activity of the ventromedial prefrontal cortex (Neider, Pace-Schott, Forselius, Pittman, & Morgan, 2011). In lucid dreamers the effectiveness of the executive functions controlled by the fronto-cortical areas were also shown in a study (Blagrove, Bell, & Wilkinson, 2010) where they were found to have better attention skills and to perform with shorter reaction times in the Stroop Test in case of incongruent stimuli. Nightmares tend to engage the dreamer into realistic, threatening events typically lacking the dreamer’s awareness and leaving their self-representation ineffective, unable to control the dream events. On the contrary, lucid dreaming is associated with elevated levels of self-awareness (Voss, Holzmann, Tuin, & Hobson, 2009), control, and higher order cognitive skills (Kahan & LaBerge, 1994). In this view the two phenomena might represent extremes of a self-awareness and self-effectiveness continuum, with regular dreams occurring in between the two extremes. In the case of lucid dreamers it is hypothesized that attentional skills are required to perform such self-awareness (Blagrove et al., 2010), and that nightmare sufferers could be characterized by impaired prefrontal and fronto-limbic functions (Simor et al., 2012). In both cases these functions in REM sleep are hypothesized to be reflected in waking neuropsychological tests.
Although developmental studies indicate that both the characteristics of dreaming and also cognitive and emotional performance gradually develop with age, the associations between dream content and cognitive development are seldom studied. Having explored the descriptive developmental patterns of children’s dreams (Sándor et al., 2015), this study (using the same database) aims to contribute to the growth of this relatively unknown field by investigating correlations between specific dream content and measures of maturational level of frontal executive functioning and intelligence variables measured by various tools. The Stroop Test, Emotional Stroop Test, and the Attention Network Test (ANT) are valid tools for measuring executive attention and fronto-limbic performance in a complex form. These tests are used to shed light onto the possible associations of executive abilities and aspects of dreaming development. In other words we aim to depict the associations between individual differences in neurocognitive maturation as revealed by performance measures in different neuropsychological tests and the formal and content related characteristics of children’s dreams.
Hypotheses
Based on the above direct and indirect evidence suggesting the relationship between the developments of cognitive functions and dreaming we hypothesize the following:
- Dream recall frequency is expected to show a positive correlation with visuospatial skills. The length of dream records is hypothesized to correlate with verbal abilities and memory capacity.
- Dream features that go through certain age-related development (human characters, actions, interactions) are expected to be directly positively associated with cognitive maturation as quantified by the performance indexes in neuropsychological tests (Fruit Stroop Test, ANT) measuring executive functions. Verbal actions in dreams are expected to correlate with verbal abilities. The quality of interactions (friendly or aggressive) is expected to associate with the Emotional Stroop measures.
- We expect bizarreness in dreams to show a positive correlation with the maturation of general intelligence and executive functions (Fruit Stroop Test, ANT: Conflict network).
- We expect the activity and agency of the self and conscious presence in the dreams to be related to executive functioning (Fruit Stroop Test, ANT: Conflict network) including the control of emotional interference (Emotional Stroop Test). In case of consciousness in the dreams we also expect an association with measures of general intelligence.
Methods
Subjects
Participants were 40 children between the ages of 4 to 8.5 years (mean: 6.3, SD: 1.6) and their parents recruited from different schools and kindergartens in Budapest. Convenience and snowball sampling was used. All of the children were from a middle class, educated environment with at least one of the parents holding a degree in higher education.
All the children were healthy; any diagnosis of mental or physical illness caused an exclusion from the study. Written consent forms were obtained from the parents and verbal consent was elicited from the children. Ethical approval of the study was received from the Semmelweis University Ethical Review Board.
Measures and procedures
The parents and children were subjected to an initial interview where they were informed about the schedule and had the opportunity to consent to the study. Dreams were obtained from the children upon morning awakenings in their homes over a 6 week period in the form of a structured dream interview. Tests of intelligence, executive functioning, and of emotional maturation were carried out in a laboratory environment upon three occasions. The order of the tasks was fixed for all subjects. Findings in relation to the maturation of dreaming were reported elsewhere (Sándor et al., 2015).
The dream collection procedure
In order to avoid unsuitable environment and nighttime awakenings which might affect especially young children’s dream reports, we developed a home-based method of dream collection with an attempt to control for the well-known methodological flaws.
Firstly, the parents were trained how to use the structured dream interview developed for this study and how to avoid suggestive questioning. Dreams were obtained from the children upon morning awakenings over a 6 week period of time in form of a structured dream interview conducted by the pre-trained parents. The 6 week-period was considered to be long enough to provide a representative sample of the children’s dreams. In order to meet the children’s possible need for extra attention in the morning we asked the parents to carry out a 5-minute conversation after waking, even if no dream was recollected.
Interviews were carried out within the first 20 minutes of the waking state each morning and were tape recorded in order to allow retrospective control over the conversation. Thus the children’s dream reports were directly heard by the researchers.
In order to rule out parental suggestions and waking fantasy penetrations from entering the reports, we introduced a 3-step control system on the dream collection and evaluation process which included control of the child’s narrative by the parent (1) and the researcher (2), and control of parental influence by the researcher (3). Dreams possibly containing more that 50% of waking fantasy elements and answers influenced by parental suggestions were discarded from the research (for more details see: Sándor et al., 2015).
Measures describing the dream reports
Words relevant to the dream narrative were counted by two independent researchers according to the rules described by Foulkes and Shepherd (1971). Word count was referred to as dream report length in the analysis.
In our developmental dream content analysis system (Sándor et al., 2015) we considered two popular content analysis methods: one developed by Foulkes and Shepherd (Foulkes & Shepherd, 1971) and the widely used system of Hall and Van de Castle (Hall & Van de Castle, 1966). For the correlational analysis we choose those dream characteristics that proved by laboratory and home studies to change significantly through development (Foulkes, 1982, 1999; Sándor et al., 2015).
Definitions:
Human character: is someone physically present in the dream, or whom the dreamer interacts with in the dream. The dreamer themselves were not counted as human characters; they were only coded as self-representation. The variable used in this study is the number of human characters per dream.
Self-initiated actions: any activity actively performed by a character is scored here. For example: “I reached for the cup…” or “I went to my grandma’s place”. The variable used in this study is the ratio of self-initiated actions per all actions.
Gross-motor activities: are those that involve the movement of whole body or a significant part of the character’s body. The variable used in the present study is the number of gross-motor activities per dreams.
Social interactions: could be aggressive (any hostile or offensive act towards a character) or friendly (any friendly or helpful overture towards another character). The variable used in this study is the number of interactions per dreams.
Bizarreness: bizarre elements were coded according to the coding method of Revonsuo and Salmivalli (1995).
Undefined setting: a setting that is mentioned in the dream report but cannot be specifically characterized by the dreamer. The variable used in this study is the number of dreams with undefined settings per all dreams with any kind of setting.
Indices of self- effectiveness:
- a) Active self-representation: coded if the dreamer is present and actively takes part in the dream mentation (e.g.: “I was on a ship” or “I made a cake”). Passive self-representation is scored if the dreamer merely views the scene, and no self-representation is coded if the dreamer is not mentioned at all. The variable used in this study is the number of dreams with an active self per all dreams.
- b) Dreamer involved success percent: calculated by dividing the number of dreamer involved successes by the sum of the dreamer involved successes and failures (Domhoff, 1999).
- c) Self-negativity percent: calculated by adding up the incidences of dreamer as a victim plus dreamer involved misfortune plus dreamer involved failure divided by all the above plus dreamer as befriended plus dreamer involved good fortune and dreamer involved success (Domhoff, 1999).
- d) Dreamer involved strivings and success: calculated by adding up the dreamer involved strivings (failures and successes) and taking the dreamer involved successes respectively and dividing them by the number of dreams for each of the children.
Cognitively reflective verbs: mental or intellectual activity of any sort. For example thinking, planning, counting, decision making, imagination, forgetting, remembering etc. Negative examples are also scored. For example: “I did not remember the way home…”. The variable used in this study is the sum of the dreams with cognitively reflective verbs per all dreams.
All of the above categories (except for the ones self-rated by the dreamer) were coded by two independent raters. For measuring inter-rater reliability we used Cohen’s Kappa which varied between .7–.87 amongst the content analysis categories.
Measures of intelligence
We used three subtests from the Wechsler Intelligence Scale for Children (WISC-IV) (Wechsler, 2003); the Vocabulary Subtest for assessing verbal abilities, the Digit Span Subtest for assessing working memory capacity and the Block Design Subtest for estimating visuospatial abilities.
Raven’s Coloured Progressive Matrices (CPM, Raven, Court, & Raven, 1995) were used to assess fluid intelligence based on non-verbal reasoning.
Measures of executive functioning
The relatively recent but well studied child Attention Network Test (child ANT, Fan et al., 2002; Rueda et al., 2004) was used to measure reaction times and precision in different components of attention: the alerting, orienting, and conflict networks. It was built on the basis of the flanker test (Eriksen & Eriksen, 1974) but uses cues to measure alertness and orienting. In the children’s version the target is a fish and children are instructed to feed the hungry fish (are asked to push a key corresponding to the direction of the target fish’s mouth as quickly as possible). The target fish is either alone or in the company of distractor fish (the target is always the central fish) (Rueda et al., 2004). Each trial begins with a central fixation cross. On congruent trials the target fish faces in the same direction as the flanking fish, on incongruent trials in the opposite direction, and on neutral trials the target fish appears alone. The appearance of the target is preceded by one of four warning cues: central cue, a double cue, a spatial cue or no cue (see Rueda et al., 2004). The children were instructed and trained before the task as described in the work of Rueda et al. (2004).
Measures based on differences in reaction times were calculated. The Alerting Network was calculated taking the average reaction time of no cue condition minus that of the double cue condition. The Orienting Network was calculated by subtracting the average reaction time of the spatial cue trials from that of the central cue trials. The Conflict Network measure was defined as the difference between reaction times for the incongruent and that of the congruent trials.
The Modified Fruit Stroop and Emotional Stroop Tests for children were used to measure inhibitory control (Archibald & Kerns, 1999), emotional interference control (Eschenbeck, Kohlmann, Heim-Dreger, Koller D., & Leser, 2004), and dimensions of executive functioning in a situation of interfering stimuli. The Fruit Stroop Test is based on the same principles as the original adult version but the congruent and incongruent stimuli are non-verbal and better suited for children as they are represented by fruits in different colours consistent or inconsistent to the target fruit (Archibald & Kerns, 1999).
The Emotional Stroop Test is a well-used tool to investigate attentional bias and emotional interference caused by emotionally salient stimuli (MacLeod, Mathews, & Tata, 1986). The task involves the presentation of neutral and emotionally loaded stimuli with different colours, and participants are asked to press the button corresponding to the colour of the stimulus as quickly as possible. Although the original version consists of neutral and emotionally charged words as stimuli, emotional facial expressions has been proven as ecologically valid stimuli (Honk, Tuiten, de Haan, vann de Hout, & Stam, 2001) and has been used with children as well as with adults (Eschenbeck et al., 2004). Since the only relevant stimulus in this task is the colour information, subjects have to suppress other perceptual information about the face shown. The shift of attention and the regulation of evoked emotions might demand extra cognitive effort from the subjects. This theory explains a slowdown in reaction time as a result of emotional stimuli especially for subjects with emotional processing difficulties (e.g. anxiety) (Eschenbeck et al., 2004). It has been shown in children that, unlike in adults, error rates as well as reaction times could be predictive for emotional disturbances such as non-clinical anxiety (Eschenbeck et al., 2004). Brain imaging studies indicate that the Emotional Stroop Test is associated with enhanced activation in the amygdala and the anterior cingulate cortex (Bremner et al., 2004; Etkin, Egner, Peraza, Kandel, & Hirsch, 2006; Whalen et al., 1998).
We used a computerized version of the Stroop Tests appearing on a 15-inch monitored laptop computer, with coloured arrow buttons in the right hand corner of the keyboard. A session of the combined Stroop Test included a familiarization phase, a trial phase and a test phase. The test phase consisted of four experimental blocks of stimuli. The first block contained 16 trials of congruent fruits followed by 16 trials of incongruent fruits in a fixed random order. The second block contained 16 happy faces and 16 angry faces in a fixed random order. The third and fourth blocks repeated the first and the second blocks.
We used a block design for the Stroop tasks in order to account for the slow effect of the negative emotional stimuli in the emotional section of the test (McKenna & Sharma, 2004). We also used a second version of the test which presented the blocks of negative/incongruent stimuli before the positive/congruent ones thus addressing the possible reaction time differences due to exhaustion of the children. These two conditions were counterbalanced between the children.
Since according to our pilot study 4 year-olds had difficulties following the instructions of pressing the original colour of the incongruently coloured stimuli, we altered the usual instruction for the stroop test and the children were asked to press the colour that they actually see and try to suppress the stimulus (the incongruent fruit) which is interfering with it, thus making the exercise similar in nature to the emotional stroop task. The interference effect is proven to be present in the case of naming the presented colour of incongruent fruit stimuli (Ménard-Buteau & Cavanagh, 1984) and in the present case reaction times and accuracy rates were also found to be higher in the case of incongruent stimuli compared to congruently coloured fruits (paired t-test, t= 6.4, p< .001 and t= 3, p< .01).
A measure based on reaction times was calculated for both the fruit Stroop and the emotional Stroop conditions. The Incongruency Index (II) was calculated by subtracting the average reaction time of the congruent stimuli from that of the incongruent stimuli. The Emotional Interference Index (EII) was calculated by subtracting the average reaction times of happy faces from that of the angry faces. In case of the Fruit Stroop Test a larger difference in reaction times shows difficulty using inhibitory control. In the case of the Emotional Stroop Test a larger difference shows slower processing of negative emotional stimuli. The accuracy measures of incongruent and negative emotional conditions were recorded.
Statistical analysis
Since the number of observations (dream reports) per child varied greatly across the sample, dream content characteristics were relativized (item/dream) for each child. These were the units of the statistical analyses unless defined otherwise in the methods section. Since the dream related variables did not meet the conditions for parametric testing (most of the dream related variables do not follow normal distribution) we used the Kendall tau (τ) method to explore the correlations between the dream characteristics of the children and their intelligence and executive measures. Age was held constant at all of the correlations presented and the degree of freedom equals to 38 in all correlations. In order to control for Type I errors, we used the Benjamini-Hochberg (B-H) correction method to adjust p values, using a 10% false discovery rate as recommended by McDonald ( 2014).
Results
Dream recall frequency and report length
In order to produce results comparable with previous studies on dream recall frequency and visuospatial skills (Foulkes, 1982, 1999), we performed correlational analysis of dream recall frequency and the Block Design Subtest (WISC), both with and without age control. The number of recalled dreams per child did not show any significant association with visuospatial abilities with either of the above methods.
Similarly, report length did not show associations with either measures of verbal or memory performance (see Table 1. for a summary of all correlations).
Figure 1. Actions and interactions in the dreams plotted against executive control measured by the modified Fruit Stroop test. All plots are controlled for age by running linear regressions with x and y variables as dependent and age as independent variable and plotting the residuals from both regressions against each other. Upper left: association of dreamer initiated actions per all actions and executive control measured by the Incongruency Index (II) of the Stroop Test. Upper right: correlation of gross-motor activities per dreams and Stroop Test Incongruency Index (II). Lower left: the number of gross-motor activities per dreams also correlates with accuracy in the Stroop Test in case of incongruent stimuli. Lower right: association between the number of interactions per dreams and Incongruency Index (II) of the Stroop Test.
Human characters, actions and interactions in the dreams
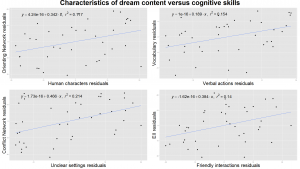
The number of human characters per dreams showed no correlations with the executive measures of either of the neuropsychological tests. On the contrary we found a positive association with the effectiveness of the Orienting Network (τ= .23, p= .04) which is an essential measure of the ability to select the relevant stimuli in a distracting environment; however the B-H correction did not confirm the reliability of this result (Figure 2.).
The number of self-initiated actions per all actions as well as the ratio of gross motor activities in dreams were significantly associated with the Incongruency Index of the Stroop Test (τ= .26, p= .02 and τ= .24, p= .03, respectively), indicating a more efficient behavioural inhibitory control with more dreamer-involved actions (Figure 1.). In addition, gross-motor activities were associated with higher accuracy in the condition of incongruent stimuli in the Stroop Test (τ= .28, p= .01) indexing better inhibitory control functions (Figure 1.).
The number of verbal actions per dreams correlated positively with the Vocabulary Subtest of the WISC (τ= .24, p= .03, Figure 2.).
Interactions, and specifically dreamer-initiated interactions per dreams, were also associated with the behavioural inhibitory control functions measured by the Incongruency Index of the Stroop Test (τ= .23, p= .03, and τ= .22, p= .04, respectively, Figure 1.). Friendly interactions per dreams showed association with the Emotional Interference Index of the Stroop Test (τ= .24, p= .03), being positively correlated with a more efficient control of emotional interference (Figure 2.). This latter result did not remain significant after the B-H correction procedure.
Figure 2. Characteristics of dream content and wakeful cognitive skills. All plots are controlled for age. Upper left: associations between the number of human characters per dreams and the ability to select the relevant stimuli in a distracting environment measured by the Orienting Network of the Attention Network Test (ANT). Upper right: the number of verbal actions per dreams correlates with the Vocabulary Subtest of the Wechsler Intelligence Scale for Children (WISC IV). Lower left: associations between the ratio of dreams with unclear settings (compared to dreams with any kind of setting mentioned) and executive control measured by the Conflict Network of the ANT. Lower right: the number of friendly interactions per dreams associated with the ability to control emotional interference measured by the Emotional Interference Index (EII) of the Emotional Stroop test.
Dream bizarreness
We found no significant association between bizarre elements in the dreams and measures of intelligence and executive functions. However, when we analysed the above relationship without controlling for the effects of age we found comparable results with previous studies (Colace, 2010): the number of dreams with bizarreness showed a significant positive correlation with general nonverbal intelligence measured by the CPM (τ= .27, p= .018) and verbal abilities measured by the Vocabulary Subtest of the WISC IV (τ= .26, p= .021). A positive tendency also appeared with visuospatial abilities measured by the Block Design Subtest of the WISC (τ= .22, p= .059).
It is worth mentioning that the number of unclear settings (a self-defined version of a subscale of dream bizarreness measure) correlated significantly with the Conflict Network of the ANT (τ= .30, p= .00)
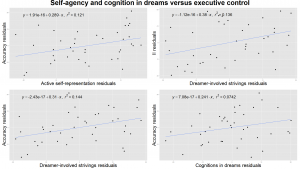
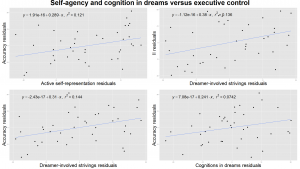
Figure 3. Self-agency and cognitive control in the dreams versus executive functioning measured by the Modified Fruit Stroop Test. All plots are controlled for age. Upper left: the ratio of dreams with active self-representation correlated with executive functioning measured by the accuracy in the Stroop Test in case of incongruent stimuli. Upper right: the number of dreamer involved strivings (dreamer’s voluntary efforts) per dreams correlated with the executive functioning measured by the Incongruency Index (II) of the Stroop Test. Lower left: dreamer involved strivings are also correlated with accuracy in the Stroop Test in case of incongruent stimuli. Lower right: the number of cognitively reflective verbs in the dreams correlated with executive efficiency measured by the accuracy in the Stroop Test in case of incongruent stimuli.
Self agency and cognition in dreams
We considered the effectiveness of the self and cognitive/metacognitive verbs in the dreams as a measure of awareness during dreaming in children. Interestingly, both the ratio of dreams with active self-representation (τ= .27, p= .03), and cognitive/metacognitive verbs (τ= .25, p= .02), together with the ratio of dreamer involved strivings (τ= .24, p= .03) showed an association with increased accuracy in the Stroop Test in case of incongruent stimuli (Figure 3.). Additionally, dreamer involved success and dreamer involved strivings per dreams were also correlated with the Incongruency Index of the Stroop Test (τ= .29, p= .01 and τ= .25, p= .02 respectively) confirming that self-effectiveness in children’s dreams is a correlate of better attentional and behavioural control (Figure 3.). Active self-representation showed a positive correlation with the accuracy measure of the Emotional Stroop Test (τ= .24, p= .03), referring to a more accurate emotional processing in the presence of negative emotions. This result was not confirmed by the Benjamini-Hochberg procedure.
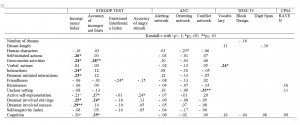
Table 1. Correlations (Kendall tau) between dream report characteristics and waking measures of neuropsychological (modified Fruit and Emotional Stroop Test for children, Attention Network Test) and intelligence (CPM, WISC IV) scores of 4-8 year-old children (n=40). Associations expected and listed in the section “Aims and hypotheses” are presented. Bold fonts represent those results that remained significant after the Benjamini-Hochberg procedure.
Discussion
The present work aims to contribute to the rare attempts in the scientific literature dealing with cognitive correlates of dreaming. Existing theoretical frameworks that consider neuro-cognitive and emotional functioning as being closely related to REM sleep and dreaming are used to discuss the results. Although the majority of the theoretical frameworks (see introduction) are based on adult dream research we conclude that examining the developmental aspects of both dreaming and cognitive functioning is a fertile direction to gain a wider multilevel perspective of dreaming (including levels of neuro-anatomic findings, cognitive and emotional functioning and behavioural aspects).
We found a positive association between wakeful verbal abilities and verbal actions in the dreams in line with our hypothesis. Since these verbal skills did not correlate with the length of the dreams, we assume that this result supports the continuity of waking abilities and dreaming rather than showing a general increase of the reports in lengths and details.
Based on previous laboratory studies (Foulkes, 1982, 1999) we hypothesized that dream recall frequency would be associated with cognitive skills, especially visuospatial abilities measured by the Block Design subtest of the WISC. Our results did not support this hypothesis; moreover dream recall frequency did not correlate with any of the cognitive measures. Similarly, in spite of direct supporting evidence in the literature (Colace, 2010; Resnick et al., 1994), we did not find significant correlation between bizarreness measured by the Revonsuo-Salmivalli system (Revonsuo & Salmivalli, 1995) and any of the cognitive measures. These divergences may be due to the differing data collection methods and settings. On-the-spot dream interviews during scheduled awakenings (in case of the laboratory study) are possibly less often affected by recall failures than the morning dream interviews, thus resulting in a possibly less biased sample of dreams and dream report rates.
Contrary to the well-defined categories of bizarreness by Revonsuo and Salmivalli (1995), the ratio of dreams reported to have an undefined setting showed a significant correlation with the effectiveness of the executive attention measured by the ANT. Undefined setting could possibly be similar to the uncertainty category of bizarreness defined by Hobson (1988) or Revonsuo and Salmivalli, except for the distinction of being reported by the dreamer. This distinction does not explain however that the well-defined and control-coded categories of bizarreness did not correlate with any measures of cognitive performance, especially that previous results show a close relationship between cognitive and behavioural control development and bizarreness measured by various bizarreness scales using dreams from school and home interviews (Colace, 2010). Since correlations presented in the work of Colace (2010) were not controlled for age we performed such correlations on our sample which proved age-control to be responsible for many of the differences. However, in our opinion age control is necessary in order to obtain a clear view on the relationship between cognitive skills and dreaming without the obvious confounder of general developmental changes.
Also based on laboratory studies that showed specific dream content characteristics as being closely correlated with age (Foulkes, 1982, 1999), we hypothesized that human characters, activities, and interactions appearing in the dreams would be associated not only with chronological age but with mental age (measured by WISC IV, ANT, Stroop, Emotional Stroop) as well. We found, although not confirmed by the B-H correction, that the number of human characters per dreams correlated with a better ability to select relevant information from the environment (orienting network), this being an essential element of attentional skills. The number of gross-motor activities and interactions in the dreams as well as the ratio of self-initiated activities turned out to be positively associated with executive attention skills, measured by the Incongruency Index of the Stroop Test. This means that more self-initiated and gross-motor activities in the dreams and more interactions between dream characters predict better functioning of the frontal executive regulation of behaviour. Our convergent findings on the activities, interactions and active self-representation in children’s dream reports (all the above features correlate with cognitive development) suggest that these content categories could be merged into a single measure of dream dynamicism. In this view we consider that the richness of the dream environment (e.g. human characters) may be a correlate of information selection skills, although this consideration remains hypothetical until further confirmation. The busyness or dynamic nature of the dreams (e.g. activities, interactions, etc.), especially if it is connected to the effort of the dreamer’s self, is possibly a correlate of executive attention skills.
Considering an extended theoretical framework, the presence of interactions in these children’s dreams may refer to an access to theory of mind capacities during dreaming (McNamara, McLaren, & Durso, 2007), which is related to managing relationships in general. Interestingly, both the number of interactions in the dreams (found in this study) and theory of mind performance (Carlson & Moses, 2001) are connected to frontal inhibitory control which is dynamically developing during the preschool and early school years (Friedman & Leslie, 2004; Magrabi, 2010). In the present study we found indirect evidence to support the possible reflection of theory of mind functions in the dream narratives: executive inhibition was correlated with the amount of cognitive and metacognitive verbs in the dreams, this variable (indicating self-reflectiveness) could also be viewed as an indicator of developing theory of mind functions. Another example that could be interpreted as an indirect support for the theory of mind skills in dreams is the presence of friendly interactions, since these possibly involve an understanding of the inner motifs, wishes and goals of the self and the other. Although not confirmed by the B-H procedure, the number of friendly interactions per dreams was associated with quicker and more efficient processing of negative emotional stimuli, which implies the association of fronto-limbic performance with attitude related dream content. Unfortunately, in this study we did not measure theory of mind skills, but future studies should consider exploring possible connections with dream characteristics, especially cognitive presence, interaction types and possibly variables of emotional presence in the dreams.
An interesting aspect of self-awareness and cognitive/metacognitive presence in the dreams is that these variables can be measured as a content of the dream reports yet they are also defining factors of lucid dreaming. By definition lucid dreaming involves higher order cognitive skills (Kahan & LaBerge, 1994) and reflective self-awareness (Voss et al., 2009) during REM sleep and is also associated with the development of cognitive functions, such as abstract thinking and cognitive insight (Voss, Frenzel, Koppehele-Gossel, & Hobson, 2012). Although one study found that the occurrence of lucid dreaming is higher during childhood and decreases after reaching young adulthood (Voss et al., 2012), differences in cognitive functioning were only assessed and found between lucid dreamers and controls in adulthood (Blagrove & Hartnell, 2000). This study showed lucid dreamers to have better attentional skills and perform with shorter reaction times in the Stroop test. Our hypotheses were confirmed as both cognitive verbs in the dreams and different measures of self-agency were found to be correlated with more efficient executive control and attention skill measured by accuracy in the Stroop Test (in case of incongruent stimuli). These results strongly support the notion that the cognitive and self-reflective features of lucid dreaming (which can be observed to varying extents in non-lucid dreams) show a continuity with waking measures of frontal executive functioning (Blagrove et al., 2010; Schredl, 2003).
We found evidence that the the busyness or dynamic nature of the dreams (dreamer involved activities, interactions, gross-motor activities), especially if connected to the effort of the dreamer’s self, is a correlate of executive attention skills measured by the Incongruency Index (a reaction-time based measure) of the Modified Fruit Stroop Test. At the same time measures of self-agency and cognitive presence in the dreams were associated with executive functions measured by the accuracy of the reactions to the incongruent stimuli and also the reaction-time based Incongruency Index in the Modified Fruit Stroop Test. Our results so far let us infer that the more effective the child’s executive control is in waking life, the stronger their presence is in dreams (manifested in activities, interactions, self-effectiveness, wilful effort and cognitive reflections). On the other hand results in connection with emotional processing were not confirmed by the B-H correction. The efficiency of emotional processing correlated with friendly interactions and active self-representation only before the correction. These results would lead us to conclude that emotional processing may be reflected in the quality and content of the above mentioned activities and interactions, but this hypothesis certainly needs more systematic support.
On the whole, while some results of this study are contributing to the mapping of the connections between brain development and its behavioural correlates during sleep and wakefulness, others raise questions about the reliability and generalizability. Contrary to what we predicted based to the literature the two kinds of measures of executive functioning (Incongruency Index of the Stroop and Conflict Network of the ANT) did not overlap in terms of dream correlates (see Table 1.). Perhaps the changes in the assessment rules of the Fruit Stroop Test we made had an effect on this outcome. However, since in the Modified Fruit Stroop the incongruency was still present (similarly to the Emotional Stroop), the Stroop effect was still measurable (see methods section), and the hypothesized associations between executive functions and dreaming were usually found, we consider this to be a reasonable alteration. In any case the issue of the correlation between dreaming and the different measures of executive functioning in children highlights the need for more specific research in this field.
Investigating dream experiences of children includes numerous methodological difficulties. We have addressed some of the problems, such as those caused by the laboratory environment or night-time awakenings by developing a new home-based methodology with control steps for eliminating the penetration of wakeful fantasy and parental suggestions. In spite of our efforts there could be several aspects of the parent –child relationship and the home environment that could potentially bias the results (more details: Sándor et al., 2015).
Other aspects that remain unclear for example is to what extent dream reports collected upon morning interviews would be representative of the general dream life of the children. Because of the morning recalls we have no means of knowing whether a dream was experienced during REM or NREM sleep, which may be relevant regarding content issues. For example McNamara et al. (2007) found that interaction-types and the role of self could be significantly different in REM versus NREM dream reports.
In order to control for the numerous correlations in this study, we used the Benjamini-Hochberg procedure. However correction of false positives with smaller sample sizes might be problematic because it may eliminate valid statistical results. Given that dream research is a field that is still at a descriptive stage, it could stifle the exploration of new, unexpected findings (Domhoff & Schneider, 2015). Instead of correction formulas some authors advocate follow up and replication studies. Considering the above reasons we presented the results with and without the Benjamini-Hochberg control throughout the article.
Keeping in mind the above constraints and limitations, this study represents an important step towards bridging the gap between adult and developmental dream research, and also between cognitive functioning in different mental states in children. It is suggested that the dynamic nature, self-agency and environmental richness of the dreams could be of special importance for further research in correlation with cognitive development. Future works should also consider the emotional aspects of dreaming and its possible roles in waking emotional processing and coping.
Conflict of Interest
The authors have no conflict of interest to declare.
Acknowledgements
The authors thank Katinka Kertész, MA (Faculty of Education and Psychology, Eötvös Lóránd University, Budapest, Hungary) for her contribution to the dream content analysis process, Klára Soltész-Várhelyi, Msc (Faculty of Humanities and Social Sciences, Pázmány Péter Catholic University, Budapest, Hungary) for programming the neuropsychological tests, and Stephen Cobeldick, MSc (Faculty of Engineering, University of Bristol, UK) for his contribution in developing an automated system for creating a data table from raw counts of dream variables.
The work was supported by the 2010 Research Grant of the BIAL Foundation (55/10) and the Hungarian National Scientific Research Fund (OTKA-K105367).
References
Adolphs, R., Tranel, D., Damasio, H., & Damasio, A. (1995). Fear and the human amygdala. Journal of Neuroscience, 15(9), 5879–5891.
Archibald, S. J., & Kerns, K. A. (1999). Identification and Description of New Tests of Executive Functioning in Children. Child Neuropsychology, 5(2), 115–129. doi:10.1076/chin.5.2.115.3167
Blagrove, M., Bell, E., & Wilkinson, A. (2010). Association of lucid dreaming frequency with Stroop task performance. Dreaming, 20(4), 280–287.
Blagrove, M., & Hartnell, S. J. (2000). Lucid dreaming: associations with internal locus of control, need for cognition and creativity. Personality and Individual Differences, 28(1), 41–47. doi:10.1016/S0191-8869(99)00078-1
Braun, A. (1997). Regional cerebral blood flow throughout the sleep-wake cycle. An H2(15)O PET study. Brain, 120(7), 1173–1197. doi:10.1093/brain/120.7.1173
Bremner, J. D., Vermetten, E., Vythilingam, M., Afzal, N., Schmahl, C., Elzinga, B., & Charney, D. S. (2004). Neural correlates of the classic color and emotional stroop in women with abuse-related posttraumatic stress disorder. Biological Psychiatry, 55(6), 612–20. doi:10.1016/j.biopsych.2003.10.001
Bulkeley, K., Broughton, B., Sanchez, A., & Stiller, J. (2005). Earliest remembered dreams. Dreaming, 15(3), 205–222.
Carlson, S. M., & Moses, L. J. (2001). Individual Differences in Inhibitory Control and Children’s Theory of Mind. Child Development, 72(4), 1032–1053. doi:10.1111/1467-8624.00333
Cartwright, R. (2011). Dreaming as a mood regulation system. In M. H. Kryger, W. C. Dement, & T. Roth (Eds.), Principles and practice of sleep medicine (5th ed.). St. Louis, Missouri: Elsevier, Saunders.
Cartwright, R., Agargun, M. Y., Kirkby, J., & Friedman, J. K. (2006). Relation of dreams to waking concerns. Psychiatry Research, 141(3), 261–270. doi:10.1016/j.psychres.2005.05.013
Cartwright, R., Luten, A., Young, M., Mercer, P., & Bears, M. (1998). Role of REM sleep and dream affect in overnight mood regulation: a study of normal volunteers. Psychiatry Research, 81(1), 1–8. doi:10.1016/S0165-1781(98)00089-4
Cicogna, P. C., Occhionero, M., Natale, V., & Esposito, M. J. (2007). Bizarreness of size and shape in dream images. Consciousness and Cognition, 16(2), 381–90. doi:10.1016/j.concog.2006.06.006
Cohen, D. B., & MacNeilage, P. F. (1974). A test of the salience hypothesis of dream recall. Journal of Consulting and Clinical Psychology, 42(5), 699–703.
Colace, C. (2003). Dream Bizarreness Reconsidered. Sleep and Hypnosis, 5(3), 105–128.
Colace, C. (2010). Children’s Dreams: From Freud’s Observations to Modern Dream Research (1st ed.). London: Karnac Books Ltd.
Corbetta, M., Kincade, J. M., Ollinger, J. M., McAvoy, M. P., & Shulman, G. L. (2000). Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nature Neuroscience, 3(3), 292–7. doi:10.1038/73009
Corbetta, M., & Shulman, G. L. (2002). Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience, 3(3), 201–215.
Coull, J. T., Frith, C. D., Frackowiak, R. S. J., & Grasby, P. M. (1996). A fronto-parietal network for rapid visual information processing: a PET study of sustained attention and working memory. Neuropsychologia, 34(11), 1085–1095. doi:10.1016/0028-3932(96)00029-2
Dang-Vu, T. T., Desseilles, M., Peigneux, P., & Maquet, P. (2006). A role for sleep in brain plasticity. Pediatric Rehabilitation,9(2),98-118.
Despert, L. J. (1949). Dreams in Children of Preschool Age. Psychoanalytic Study of the Child, 3, 141–180.
Domhoff, B. (1969). Home Dreams versus Laboratory Dreams: Home Dreams are better. In Kramer (Ed.), Dream psychology and the new biology of dreaming (pp. 199–217).
Ilinois: Charles C Thomas, Springfield.
Domhoff, G. W. (1999). New directions in the study of dream content using the Hall and Van de Castle coding system. Dreaming, 9(2-3), 115–137.
Domhoff, G. W. (2001). A New Neurocognitive Theory of Dreams. Dreaming, 11(1), 13–33. doi:10.1023/A:1009464416649
Domhoff, G. W. (2011). The neural substrate for dreaming: is it a subsystem of the default
network? Consciousness and Cognition, 20(4), 1163–74. doi:10.1016/j.concog.2011.03.001
Domhoff, G. W., & Kamiya, J. oe. (1964). Problems in Dream Content Study With
Objective Indicators II. Appearance of Experimental Situation in Laboratory Dream
Narratives. Arch Gen Psychiatry, 11(5), 525–528.
Domhoff, G. W., & Schneider, A. (2015). Correcting for multiple comparisons in studies of dream content: A statistical addition to the Hall/Van de Castle coding system. Dreaming, 25(1), 59–69
Eriksen, B. A., & Eriksen, C. W. (1974). Effects of noise letters upon the identification of a target letter in a nonsearch task. Perception & Psychophysics, 16(1), 143–149. doi:10.3758/BF03203267
Eschenbeck, H., Kohlmann, C. W., Heim-Dreger, U., Koller D., & Leser, M. (2004). Processing bias and anxiety in primary school children:A modified emotional Stroop colour-naming task using pictorial facial expressions. Psychology Science, 46(4), 451–465.
Etkin, A., Egner, T., Peraza, D. M., Kandel, E. R., & Hirsch, J. (2006). Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron, 51(6), 871–82. doi:10.1016/j.neuron.2006.07.029
Fan, J., Flombaum, J. I., McCandliss, B. D., Thomas, K. M., & Posner, M. I. (2003). Cognitive and Brain Consequences of Conflict. NeuroImage, 18(1), 42–57. doi:10.1006/nimg.2002.1319
Fan, J., McCandliss, B. D., Sommer, T., Raz, M., Posner, M. I., & Raz, A. (2002). Testing the efficiency and independence of attentional networks. Journal of Cognitive Neuroscience, 14(3), 340–7. doi:10.1162/089892902317361886
Fan, J., & Posner, M. (2004). Human attentional networks. Psychiatrische Praxis, 31(Supplement 2), S210– s214.
Feinstein, J. S., Adolphs, R., Damasio, A., & Tranel, D. (2011). The human amygdala and the induction and experience of fear. Current Biology : CB, 21(1), 34–8. doi:10.1016/j.cub.2010.11.042
Foulkes, D. (1979). Home and laboratory dreams: four empirical studies and a conceptual reevaluation. Sleep, 2(2), 233–51
Foulkes, D. (1982). Children’s Dreams: Longitudinal Studies. John Wiley & Sons Inc.
Foulkes, D. (1999). Children’s Dreaming and the Development of Consciousness. Cambridge, Massachusetts: Harvard University Press.
Foulkes, D., Hollifield, M., Sullivan, B., Bradley, L., & Terry, R. (1990). REM Dreaming and Cognitive Skills at Ages 5-8: A Cross-sectional Study. International Journal of Behavioral Development, 13(4), 447–465. doi:10.1177/016502549001300404
Foulkes, D., & Shepherd, J. (1971). Manual for a Scoring System for Children’s Dreams. Laramie: University of Wyoming.
Friedman, O., & Leslie, A. M. (2004). Mechanisms of belief-desire reasoning. Inhibition and bias. Psychological Science, 15(8), 547–52. doi:10.1111/j.0956-7976.2004.00717.x
Hall, C. S., & Van de Castle, R. L. (1966). The Content Analysis of Dreams. New York: Appleton-Century-Crofts.
Hänsel, A., & von Känel, R. (2008). The ventro-medial prefrontal cortex: a major link between the autonomic nervous system, regulation of emotion, and stress reactivity? BioPsychoSocial Medicine, 2, 21. doi:10.1186/1751-0759-2-21
van der Helm, E., Yao, J., Dutt, S., Rao, V., Saletin, J. M., & Walker, M. P. (2011). REM
sleep depotentiates amygdala activity to previous emotional experiences. Current Biology,
21(23), 2029–32. doi:10.1016/j.cub.2011.10.052
Hobson, J. A. (1988). The dreaming brain. New York: Basic books.
Honig, A. S., & Nealis, A. L. (2012). What do young children dream about? Early Child Development and Care, 182(6), 771–795. doi:10.1080/03004430.2011.579797
Honk, J. Van, Tuiten, A., de Haan, E., vann de Hout, M., & Stam, H. (2001). Attentional biases for angry faces: Relationships to trait anger and anxiety. Cognition & Emotion, 15(3), 279–297. doi:10.1080/02699930126112
Jenni, O. G., & Dahl, R. E. (2008). Sleep, cognition, and emotion: A developmental view. In C. A. Nelson & M. Luciana (Eds.), Handbook of developmental cognitive neuroscience (2nd ed.) (pp. 807–817). Cambridge: MIT Press.
Kahan, T. L., & LaBerge, S. (1994). Lucid Dreaming as Metacognition: Implications for Cognitive Science. Consciousness and Cognition, 3, 246–264.
Levin, R., & Nielsen, T. ore. (2007). Disturbed Dreaming, Posttraumatic Stress Disorder, and Affect Distress: A Review and Neurocognitive Model. Psychological Bulletin, 133(3), 482–528.
MacDonald, A. W. (2000). Dissociating the Role of the Dorsolateral Prefrontal and Anterior Cingulate Cortex in Cognitive Control. Science, 288(5472), 1835–1838. doi:10.1126/science.288.5472.1835
MacLeod, C., Mathews, A., & Tata, P. (1986). Attentional bias in emotional disorders. Journal of Abnormal Psychology, 95(1), 15–20.
Magrabi, A. (2010). Theory of Mind and Executive Functions. University of Osnabrueck.
Maquet, P., Péters, J., Aerts, J., Delfiore, G., Degueldre, C., Luxen, A., & Franck, G. (1996). Functional neuroanatomy of human rapid-eye-movement sleep and dreaming. Nature, 383(6596), 163–6. doi:10.1038/383163a0
McDonald, J. H. (2014). Handbook of Biological Statistics (3rd editio.). Baltimore, Maryland: Sparky House Publishing.
McKenna, F. P., & Sharma, D. (2004). Reversing the Emotional Stroop Effect Reveals That It Is Not What It Seems: The Role of Fast and Slow Components. Journal of Experimental Psychology: Learning, Memory, and Cognition, 30(2), 382–392.
McNamara, P., McLaren, D., & Durso, K. (2007). Representation of the Self in REM and NREM Dreams. Dreaming, 17(2), 113–126.
Ménard-Buteau, C., & Cavanagh, P. (1984). Localisation de l’interférence forme/couleur au niveau perceptuel dans une tâche de type Stroop avec des stimuli-dessins. / Localization of form/color interference at the perceptual level in a Stroop task with drawings. Canadian Journal of Psychology/Revue Canadienne de Psychologie, 38(3), 421–439.
Mirmiran, M. (1995). The function of fetal/neonatal rapid eye movement sleep.
Behavioural Brain Research, 69(1-2), 13–22. doi:10.1016/0166-4328(95)00019-P.
Muzur, A., Pace-Schott, E. F., & Hobson, J. A. (2002). The prefrontal cortex in sleep. Trends in Cognitive Sciences, 6(11), 475–481. doi:10.1016/S1364-6613(02)01992-7
Neider, M., Pace-Schott, E. F., Forselius, E., Pittman, B., & Morgan, P. T. (2011). Lucid dreaming and ventromedial versus dorsolateral prefrontal task performance. Consciousness and Cognition, 20(2), 234–44. doi:10.1016/j.concog.2010.08.001
Nielsen, T., & Levin, R. (2007). Nightmares: A new neurocognitive model. Sleep Medicine Reviews, 11(4), 295–310. doi:10.1016/j.smrv.2007.03.004
Nofzinger, E. A., Mintun, M. A., Wiseman, M., Kupfer, D. J., & Moore, R. Y. (1997). Forebrain activation in REM sleep: an FDG PET study. Brain Research, 770(1-2), 192–201. doi:10.1016/S0006-8993(97)00807-X
Nofzinger, E. A., & Maquet, P. (2011). What Brain Imaging Reveals about Sleep Generation and Maintenace. In M. H. Kryger, W. C. Dement, & T. Roth (Eds.), Principles and practice of sleep medicine (5th ed., pp. 201–214). St. Louis, Missouri: Elsevier, Saunders.
Oberst, U., Charles, C., & Chamarro, A. (2005). Influence of gender and age in aggressive dream content of Spanish children and adolescents. Dreaming, 15(3), 170–177. doi:10.1037/1053-0797.15.3.170
Raven, J., Court, J., & Raven, J. C. (1995). Manual for Raven’s progressive matrices and vocabularyscales. Oxford: Oxford Psychologists Press.
Resnick, J., Stickgold, R., Rittenhouse, C. D., & Hobson, J. A. (1994). Self-representation and bizarreness in children’s dream reports collected in the home setting. Consciousness and Cognition, 3, 30–45.
Revonsuo, A., & Salmivalli, C. (1995). A content analysis of bizarre elements in dreams. Dreaming, 5(3), 169–187.
Rueda, M. R., Fan, J., McCandliss, B. D., Halparin, J. D., Gruber, D. B., Lercari., L. P., & Posner, M. I. (2004). Development of attentional networks in childhood. Neuropsychologia, 42, 1029–1040.
Sándor, P., Szakadát, S., & Bódizs, R. (2014). Ontogeny of dreaming: a review of empirical studies. Sleep Medicine Reviews, 18(5), 435–449. doi:10.1016/j.smrv.2014.02.001
Sándor, P., Szakadát, S., Kertész, K., & Bódizs, R. (2015). Content analysis of 4 to 8 year-old children’s dream reports. Frontiers in Psychology, 6(534). doi:10.3389/fpsyg.2015.00534
Schredl, M. (2003). Continuity between waking and dreaming: A proposal for a mathematical model. Sleep and Hypnosis, 5(1), 38–52.
Simor, P., Pajkossy, P., Horváth, K., & Bódizs, R. (2012). Impaired executive functions in subjects with frequent nightmares as reflected by performance in different neuropsychological tasks. Brain and Cognition, 78(3), 274–283.
Solms, M. (1997). The neuropsychology of dreams: A clinico-anatomical study. Lawrence Erlbaum Associates Publishers.
Solms, M. (2003). Dreaming and REM sleep are controlled by different brain mechanisms. In E. F. Pace-Schott, M. Solms, M. Blagrove, & S. Harnad (Eds.), Sleep and Dreaming Scientific Advances and Reconsiderations (Vol. 23, pp. 51–58). Cambridge University Press.
Staunton, H. (2001). The Function of Dreaming. Reviews in the Neurosciences, 12(4), 365–371.
Strauch, I. (2004). Traume im ubergang von der kindheit ins jugendalter: Ergebnisse einer
langzeitstudie. Bern: Verlag Hans Huber.
Strauch, I. (2005). REM dreaming in the transition from late childhood to adolescence: A longitudinal study. Dreaming, 15(3), 155–169. doi:10.1037/1053-0797.15.3.155
Strauch, I., & Meier, B. (1996). In search of dreams: Results of experimental dream research. New York: State University of New York Press.
Urry, H. L., van Reekum, C. M., Johnstone, T., Kalin, N. H., Thurow, M. E., Schaefer, H. S., … Davidson, R. J. (2006). Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 26(16), 4415–25. doi:10.1523/JNEUROSCI.3215-05.2006
Van Veen, V., & Carter, C. S. (2005). Separating semantic conflict and response conflict in the Stroop task: a functional MRI study. NeuroImage, 27(3), 497–504. doi:10.1016/j.neuroimage.2005.04.042
Voss, U., Frenzel, C., Koppehele-Gossel, J., & Hobson, A. (2012). Lucid dreaming: an age-dependent brain dissociation. Journal of Sleep Research, 21(6), 634–42. doi:10.1111/j.1365-2869.2012.01022.x
Voss, U., Holzmann, R., Tuin, I., & Hobson, J. A. (2009). Lucid dreaming: a state of consciousness with features of both waking and non-lucid dreaming. Sleep, 32(9), 1191–200.
Walker, M. P., & van der Helm, E. (2009). Overnight Therapy? The Role of Sleep in
Emotional Brain Processing. Psychological Bulletin, 135(5), 731–748.
Wechsler, D. (2003).WISC-IV Wechsler Intelligence Scale for Children: Technical and
Interpretative Manual. Pearson.
Weisz, R., & Foulkes, D. (1970). Home And Laboratory Dreams Collected Under Uniform
Sampling Conditions. Psychophysiology, 6(5), 588–596. doi:10.1111/j.1469
8986.1970.tb02247.x
Whalen, P. J., Bush, G., McNally, R. J., Wilhelm, S., McInerney, S. C., Jenike, M. A., &
Rauch, S. L. (1998). The emotional counting stroop paradigm: a functional magnetic
resonance imaging probe of the anterior cingulate affective division. Biological Psychiatry,
44(12), 1219–1228. doi:10.1016/S0006-3223(98)00251-0