Developmental Psychology 2016, Vol.52, No.12, 2118-2129
DOI: 10.1037/dev0000233
1 Semmelweis University, Budapest Hungary
2 National Institute of Clinical Neuroscience, Budapest, Hungary
3 Pázmány Péter Catholic University, Hungary
Abstract
Sleep spindles act as a powerful marker of individual differences in cognitive ability. Sleep spindle parameters correlate with both age-related changes in cognitive abilities and with the age-independent concept of IQ. While some studies have specifically demonstrated the relationship between sleep spindles and intelligence in young children, our previous work in older subjects revealed sex differences in the sleep spindle correlates of IQ, which was never investigated in small children before. We investigated the relationship between age, Raven Colored Progressive Matrices (CPM) scores and sleep spindles in 28 young children (age 4–8 years, 15 girls). We specifically investigated sex differences in the psychometric correlates of sleep spindles. We also aimed to separate the correlates of sleep spindles that are because of age-related maturation from other effects that reflect an age-independent relationship between sleep spindles and general intelligence. Our results revealed a modest positive correlation between fast spindle amplitude and age. Raven CPM scores positively correlated with both slow and fast spindle amplitude, but this effect remained a tendency in males and vanished after correcting for the effects of age. Age-corrected correlations between Raven CPM scores and both slow and fast spindle amplitude were only significant in females. Overall, our results show that in male children sleep spindles are a maturational marker, but in female children they indicate trait-like intelligence, in line with previous studies in adolescent and adult subjects. Thalamocortical white matter connectivity may be the underlying mechanism behind both higher spindle amplitude and higher intelligence in female, but not male subjects. (PsycINFO Database Record (c) 2016 APA, all rights reserved)
Keywords: sleep spindles, Raven Colored Progressive Matrices, maturation, childhood, sex differences
Introduction
Performance in an intelligence test can be viewed as both a state and trait variable (Anderson, 2004; Geiger, et al., 2010), that is, either a measure of immediate performance affected by the level of cognitive development and environmental factors such as fatigue, or a measure of an underlying, individually stable general ability. Intelligence is conceptualized as a trait variable, that is, an ability which is subject to very little variation across the life span. However, the actual performance in intelligence tests (as measured by raw scores) increases greatly from early childhood to adulthood, and it may decline into senescence. Standard IQ scores are therefore obtained using an age-dependent standard; effectively determining trait intelligence comparing an individual to his/her similarly aged peers, resulting in a score that actually remains similar across the lifespan. Developmental studies can benefit from both approaches: using the performance in IQ tests both as a measure of mental age reflecting the actual level of neurocognitive development, but also as a measure of trait-like intelligence. The difference between the two approaches is effectively the correction for the effect of age, which is a source of crucially important variance if maturational aspects are investigated but constitutes noise in case of trait-like comparisons.
Spectral (Luigi De Gennaro, Ferrara, Vecchio, Curcio, & Bertini, 2005; Finelli, Achermann, & Borbely, 2001; Werth, Achermann, Dijk, & Borbely, 1997), macrostructural (Linkowski, Kerkhofs, Hauspie, Susanne, & Mendlewicz, 1989; Tucker, Dinges, & Van Dongen, 2007) and microstructural (Gaillard & Blois, 1981; Feinberg, 1974) characteristics of sleep EEG are also individually stable (trait-like) with a strong genetic determination (Ambrosius et al., 2008; L. De Gennaro et al., 2008; Landolt, 2011). This trait-like nature of sleep structure – along with significant inter-individual differences – is what makes sleep oscillations candidate markers for individual differences in cognitive ability.
One of the trait-like sleep features most frequently studied as a potential correlate of cognitive ability is sleep spindles. Sleep spindles are prominent thalamocortical oscillations arising in medium depth NREM sleep which lead to long-term synaptic changes including long-term potentiation (LTP) (Amzica & Steriade, 2000; Rosanova & Ulrich, 2005), drive hippocampal-cortical communication through ripples (Clemens et al., 2011; Genzel, Kroes, Dresler, & Battaglia, 2014) and may have a causal role in sleep-related memory consolidation (Stuart M. Fogel & Smith, 2011). Sleep spindles have been repeatedly shown to be associated with trait cognitive ability, that is, various measures of intelligence (Bodizs et al., 2005; S. M. Fogel, Nader, Cote, & Smith, 2007; M. Schabus et al., 2006). However, our recent findings (Bódizs, Gombos, Ujma, & Kovács, 2014; P. P. Ujma et al., 2014) shed light on a possible sexual dimorphism in the correlation between sleep spindles and intelligence: most sleep spindle measures (particularly sleep spindle amplitude) were only positively correlated with intelligence in female adolescents and adults. In contrast, the dominant oscillation frequency of the fast subtype of sleep spindles was correlated with fluid intelligence of adolescent males, but not females (Bódizs et al., 2014). Based on the association between sleep spindle amplitude and white matter integrity (Piantoni et al., 2013) the spindle-IQ relationship in adolescents and adults was interpreted as evidence for the neural architectural foundation of fluid intelligence (Bódizs et al., 2014; Ujma et al., 2014).
On the other hand, the emergence of sleep spindles follows an age-dependent pattern (Scholle, Zwacka, & Scholle, 2007; Shinomiya, Nagata, Takahashi, & Masumura, 1999; Clawson, Durkin & Aton, 2016) and was considered as a biomarker of brain function and plasticity (Urakami, Ioannides, & Kostopoulos, 2012), These associations also render sleep spindles a potential state marker of cognitive development, reflecting maturation rather than trait ability. Therefore, as sleep spindles, performance in intelligence tests and age are strongly intercorrelated, it is important to distinguish between sleep spindle parameters which are correlated with IQ in children because they reflect ageing (state marker) and which are also correlated with IQ when the effects of age are accounted for (trait marker).
The relationship between sleep and intelligence has been specifically investigated in childhood. The pioneer study of Busby and Pivik (Busby & Pivik, 1983) reported significantly more stage 2 sleep in school aged children with higher intelligence scores compared to those with scores in the normal range. Since then, several detailed sleep studies in pre-adolescent children have become available. One study with a sample of 6 female and 8 male children between 9-12 years of age (Geiger et al., 2011) found a positive association between full-scale IQ, fluid IQ (measured by the Wechsler Intelligence Scale for Children) and individually adjusted spindle power as well as a negative association with spindle peak frequency. Another study with 28 female and 35 male children (8-11 years old) also reported higher slow sleep spindle activity in children with higher IQ scores on the Wechsler Intelligence Scale for Children (WISC-IV) (Hoedlmoser et al., 2014). Two other studies (Chatburn, et al. 2013; Gruber et al., 2013) with somewhat larger samples and wider age ranges (4-12 and 7-11 years, respectively), but with a similar distribution of sex, however, failed to find such an association, at least when full-scale IQ was considered. On the other hand, Chatburn et al. (2013) did find a negative correlation between spindle frequency and performance on the Stanford-Binet nonverbal working memory task (and other neuropsychological tasks), while Gruber et al. (2013) found a similar negative association between spindle frequency and WISC perceptual reasoning index as well as working memory. These correlations generally remain significant after correcting for the effects of age, suggesting a truly trait-like association between spindle parameters and intelligence.
These studies, however, did not differentiate between slow and fast spindles with one exception (Hoedlmoser, et al., 2014), despite mounting physiological evidence for their different mechanisms and potentially different functions (Andrillon et al., 2011; M. Schabus et al., 2007). Also, while one study (Geiger, et al., 2011) identified sleep spindles based on individual EEG spectra, in the other cases sleep spindles were identified based on fixed frequency windows, despite large inter-individual variations in the width and location of the spectral peaks which are characteristic of sleep spindles (P. P. Ujma et al., 2015). While sex differences in cognitive (D. I. Miller & Halpern, 2014) and sleep spindle variables (Huupponen et al., 2002) have been reported, none of the above studies searched for a potential sexual dimorphism in the sleep spindle correlates of IQ. While generally reporting on the relationship between age and sleep spindle parameters, most of these studies did not specifically investigate which sleep spindle characteristics are state (developmental) or trait (individually stable) correlates of intelligence.
Therefore, our study aimed to investigate the association between sleep spindles and IQ based on a spindle detection method which separates slow and fast spindles and uses individually determined frequencies to detect them. We also specifically investigated whether or not the previously seen sexual dimorphism in the sleep spindle correlates of IQ (Bódizs, et al., 2014; P. P. Ujma, et al., 2014) is present in early childhood (before puberty). Furthermore, we calculated the relationship between sleep spindle parameters and intelligence test scores both with and without age correction, yielding an estimate of state and trait sleep spindle correlates of cognitive performance.
We investigated potential correlations in an age range previously not mapped: 4-8 year old children, that is, in the lower half of the age range of the earliest available study (Chatburn, et al., 2013). We consider this age range as one which merits special interest because of mounting evidence for a striking developmental progress in both neural architecture (Avants et al., 2015) and cognitive abilities including the intensive myelination of the associative cortices(Gibson & Petersen, 1991) and the increases in non-verbal reasoning ability (J. Raven, 2000). It was hypothesized that children’s improved performance with maturation might result from myelination (E. M. Miller, 1994), which is significant as white matter architecture is also a key correlate of sleep spindle activity (Piantoni et al., 2013), rendering sleep spindles a particularly good candidate index of cognitive ability and development.
Methods
We used convenience and snowball sampling for the recruitment of 33 pre-school and school aged children in the greater Budapest area. All the participants were from a middle class background with at least one parent holding a degree in higher education. Any diagnosis of mental or physical illness caused an exclusion from the study. Written consent forms were obtained from the parents. Ethical approval of the study was received from the Semmelweis University Ethical Review Board. This study is a part of a larger research project investigating the development of dreaming and sleeping in comparison with cognitive and emotional maturation.
The children underwent all-night polysomnography recordings in a sleep laboratory in the presence of their parents for two (or three in some cases) consecutive nights. Children were allowed to sleep at least 8.5 hours at will, after which the parents had the choice to wake them up or let them sleep. For this reason we report descriptive statistics on sleep duration but excluded it from correlational analyses. Due to the poor quality of second-night recordings the recording of the first night was used in case of one child (male, age 4.34 years). Due to the poor quality of both first and second night (caused by EEG apparatus’ technical problems) recordings a third night was recorded and used for analysis in the case of another four children (3 females, one male, ages 8.22 years, 6.25 years, 7.05 years and 8.5 years, respectively). In case of five additional children first and second night recordings were of poor quality but the families refused to undergo a third polysomnography recording and therefore the children were excluded. Therefore, we analyzed recordings of 1 first night, 23 second nights and 4 third nights from a total of 28 children (15 females, 13 males, age: 3.84-8.42 years). The age of the children was obtained in months and re-calculated to years including fractions which is how it is reported in this article. We aimed to reduce the number of nights spent in the sleep laboratory to the minimum necessary, and we used first and third night recordings due to the inter-night stability of the individual EEG spectrum in the typical sleep spindle frequency range even in case of drastic manipulations (Luigi De Gennaro, et al., 2005).
On both nights, subjects were fitted with 19 EEG electrodes (Fp1, Fp2, F3, F4, Fz, F7, F8, C3, C4, Cz, P3, P4, Pz, T3, T4, T5, T6, O1, O2) according to the 10–20 electrode placement system (Jasper, 1958) as well as with two EOG electrodes (bipolar channel) monitoring vertical and horizontal eye-movements; EMG electrodes (bipolar channels) for the chin muscles, two ECG electrodes according to standard lead I. Gold coated Ag/AgCl EEG cup electrodes were fixed with EC2 Grass Electrode Cream (Grass Technologies, USA) and referred to the mathematically- linked mastoids. Impedances were kept below 10 kΩ. In 26 children, signals were collected using the 32 channel EEG/polysystem (Brain-Quick BQ 132S, Micromed, Italy), prefiltered (0.33–1500 Hz, 40 dB/decade anti-aliasing hardware input filter), amplified and digitized with 4096 Hz/channel sampling rate (synchronous) with 12 bit resolution. A further 40 dB/decade anti-aliasing digital filter was applied by digital signal processing which low-pass filtered the data at 450 Hz. Finally, the digitized and filtered EEG was undersampled at 1024 Hz. Two further children were recorded in their homes by using the newly available SD-LTM 32 Express ambulatory home polysomnography device and the System Plus Evolution Software (Micromed, Italy) with the following technical characteristics: 0.15-250 Hz hardware input filtering (40 dB/decade), 4096 Hz/channel synchronous sampling rate, 22 bit resolution, downsampling (decimation) to 1024 Hz after 463.3 Hz anti-aliasing filtering performed by firmware. Because of the small amplitude attenuation due to the hardware filter characteristics of the EEG devices in the spindle frequency range data from the two recording systems were pooled without correcting for device-specific amplitude differences (P. P. Ujma, et al., 2014).
All polysomnography recordings were scored according to standard criteria (Iber, Ancoli-Israel, Chesson, & Quan, 2007) based on 20 second epochs, artifacts were manually rejected based on 4 second epochs using in-house software, FerciosEEG (© 2009-2014. Ferenc Gombos). Artifact-free N2 and SWS EEG epochs (in line with the standard application of the IAM method and the previously used methodology of our laboratory) were fed to the Individual Adjustment Method (IAM) sleep spindle detection algorithm (Bódizs, Körmendi, Rigó, & Lázár, 2009; Péter Przemyslaw Ujma, et al., 2015). This automatic detection method identified spindles based at individually determined slow and fast spindle frequencies, which were set based on the shape of the individual EEG spectrum of frontal electrodes (for slow spindles) and central/parietal electrodes (for fast spindles), respectively. Slow and fast spindle spectral peaks were detected based on the second-order derivatives of the average spectrum of the frontal electrodes (for slow spindles) and centro-parietal electrodes (for fast spindles). A spectral peak was defined between frequencies where the second-order derivatives of spectral power dipped beyond zero. If the frequency range of a spectral peak was narrower than 0.5 Hz, it was symmetrically broadened to reach at least this width. In 11 cases subjects slow spindle spectral peaks were clearly visible but sub-critical and the bulge in second-order derivatives of spectral power did not reach zero. In these subjects slow spindle spectral peaks were still defined at the edges of the bulge of second-order derivatives, in line with our previous methodology (P. P. Ujma, et al., 2014). In 4 subjects only a flattening of monotonously decreasing spectral power was visible instead of a true slow spindle peak. In these subjects, sleep spindle frequencies were determined at the edges of this flattened area. There was no uncertainty regarding fast spindles.
The IAM method calculated individual averages for slow and fast spindle frequency (Hz), density (no./minute), duration (second) and amplitude (μV, based on the envelope of the EEG signal filtered to the individual frequencies). Sleep spindle analysis was restricted to the first 8.5 hours of sleep even if a child slept more.
All children completed a psychological and neuropsychological battery, among them the Raven’s Colored Progressive Matrices (CPM), a nonverbal reasoning test and test of IQ (J. C. Raven, Raven, & Court, 1962). The CPM consists of a series of nonfigurative patterns which must be completed using spatial and color information. It was specifically developed for use in children and other subjects not assumed to possess the reasoning ability of a healthy adult. The CPM raw score was used to assess the children’s cognitive ability. Psychological measures including CPM were assessed during the day (typically from 10 to 12 AM and from 3 to 5 PM) on three separate occasions on days not preceding or following polysomnography recordings. Psychological tests were administered by an experienced psychologist in a quiet room with the parent sitting silently next door.
We investigated the correlations between CPM scores, sleep macrostructure, age and individual sleep spindle parameters using Pearson’s point-moment correlations. In order to adjust for multiple comparisons in case of sleep macrostructure parameters we used the Benjamini-Hochberg method of False Discovery Rate (Benjamini & Hochberg, 1995). For sleep spindle parameters which can be interpreted topographically we used a modified version of the Rüger area method (Abt, 1987; Bódizs, et al., 2014; Duffy et al., 1990) in order to adjust for multiple comparisons. We defined areas of potential significance on the scalp where uncorrected p-values on at least two neighboring electrodes were below the conventional significance limit (α=0.05). If the uncorrected p-values were below α/2 (p<0.025) for at least 50% of the correlations within the area of significance, then the null hypothesis was rejected for the area as a whole.
In order to assess the correlations between sleep spindles and cognitive performance both from a developmental/maturational and trait perspective (Geiger, Achermann, & Jenni, 2010), we computed the correlations between spindle parameters and CPM scores both with and without correcting for age, similarly to our previous study in an adolescent (postpubertal) sample (Bódizs, et al., 2014).
In order to obtain a better localization of regions with significant correlations between sleep spindles and age or IQ the correlations were represented by significance probability maps (Hassainia, Petit, & Montplaisir, 1994).
Results
Male and female children did not differ in their age (Meanfemale= 5.95; Meanmale=6.51; t=0.97; p>0.3) or CPM score (Meanfemale= 24.47; Meanmale=27.21; t=1.12; p>0.25). Unsurprisingly, CPM scores correlated very strongly and positively with age (r=0.776, p<0.001) without notable sex differences.
Sleep macrostructure is reported in Table 1.
Table 1.
After correcting for multiple comparisons (correction for false discovery rate using the Benjamini-Hochberg method (Benjamini & Hochberg, 1995), no macrostructural parameter was significantly correlated with intelligence. Longer NREM duration in female children and shorter sleep latency and shorter wake duration in male children was significantly associated with intelligence, but these correlations were not significant after correcting for multiple comparisons.
The frequency of sleep spindles was unusually low: fast spindle frequency typically remained under 13 Hz (which is the slow spindle range in adults) and slow spindles were characterized by an even lower frequency. While surprising at first, this is in line with other results: a descriptive study (McClain et al., 2016) found similar spindle frequencies in similarly aged children, while another one (Doucette et al., 2015) found a positive association between processing speed and 10-13 Hz – that is, much lower than adult spindle frequency – power in preschoolers. Aside from previous results, we are confident about our classification of slow and fast spindles also since we never saw spectral peaks in the conventional (13-15 Hz) fast spindle range, but we observed the following features:
1.) spindle spectral peaks were strong and unambiguous even in the <13 Hz range, which we had found characteristic for fast spindles (P. P. Ujma, et al., 2014)
2.) we unambiguously saw dual spectral peaks in 24 and with some ambiguity in four further children, and the lower spectral peaks were always below even this unusually slow fast spindle frequency range
3.) the slow spindles detected using the IAM approach had a frontal maximum amplitude, while fast spindles had a central maximum amplitude (see Table 2), in line with standard descriptions of slow and fast spindles (Stuart M. Fogel & Smith, 2011).
Table 2 shows descriptive statistics of sleep spindle parameters, including frequency.
Table 2.
Fast spindle amplitude had a tendency for a positive correlation with age in both female and male children, but after correcting for multiple comparisons this association only reached significance on Pz and P4 when the entire sample was considered (r=0.45, p=0.019 and r=0.485, p=009, respectively). No other sleep spindle parameter was significantly associated with age.
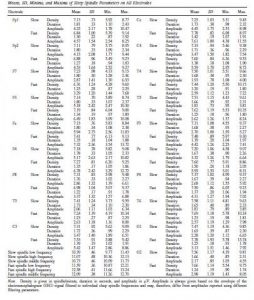
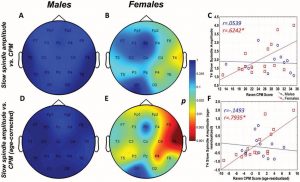
Raven CPM scores correlated positively with both slow and fast spindle amplitude in the entire sample, but this association remained a tendency in males (Figure 1 and 2, upper panels.) In female children, significant positive correlations between CPM scores and slow spindle amplitude on T4, T6 and P4 as well as fast spindle amplitude over the entire right hemisphere (Fp2, F4, C4, Cz, F8,
P4, T4, T6, O2) formed areas of significance. Furthermore, solitary correlations between CPM scores and fast spindle amplitude on Pz in males (r=0.692, p=0.021) and fast spindle amplitude on T3 in females (r=0.704, p=0.003) were found, but these did not constitute an area of significance (and the one in females was not contiguous with one). No other sleep spindle parameter was significantly associated with Raven CPM scores.
Figure 1.
Figure 1. Age-corrected and age-uncorrected correlations between slow sleep spindle amplitude and Raven Colored Progressive Matrices (CPM) scores. Significance probability maps depict the age-uncorrected (Panels A and B) and age-corrected (Panels D and E) correlations between slow spindle amplitude and CPM score in boys (Panels A and D) and girls (panels B and E). p values are plotted on an inverted logarithmic scale. Scatterplots illustrate the age-uncorrected (Panel C) and age-corrected (Panel F) correlation between slow spindle amplitude and CPM score for both sexes for the derivation where the strongest correlation was observed. The age-uncorrected and age-corrected correlation between slow spindle amplitude and CPM scores in females was statistically significant after correcting for multiple comparisons (Panels B–C and E–F). See the online article for the color version of this figure.
Figure 2.
Figure 2. Age-corrected and age-uncorrected correlations between fast sleep spindle amplitude and Raven Colored Progressive Matrices (CPM) scores. Significance probability maps depict the age-uncorrected (Panels A and B) and age-corrected (Panels D and E) correlations between fast spindle amplitude and CPM score in male (Panels A and D) and female (Panels B and E) children. p values are plotted on an inverted logarithmic scale. Scatterplots illustrate the age-uncorrected (Panel C) and age-corrected (Panel F) correlation between fast spindle amplitude and CPM score for both sexes for the derivation where the strongest correlation was observed. The age-uncorrected and age-corrected correlation between fast spindle amplitude and CPM scores in girls was statistically significant after correcting for multiple comparisons (Panels B–C and E–F). See the online article for the color version of this article.
For both areas of significance, we directly compared the correlation coefficients of the male and female subsample at the derivation where the correlation was maximal (T4 for slow spindles, P4 for fast spindles) using the Fisher r-to-z method. In both cases, the correlation coefficients were not significantly different (zslow=1.84 , pslow=0.067; zfast=1.53 , pfast=0.126).
The pattern of correlation became clearer after correcting for the effects of age in order to establish trait (not maturation-dependent) correlates of intelligence. In males, no correlation between CPM scores and spindle parameters remained significant. In females, however, the relationship between CPM scores and spindle amplitude survived correcting for the effects of age. Significant correlations with slow spindle amplitude extended over almost the entire scalp (except for Fz and O1) with a right lateral maximum. Furthermore, positive correlations with fast spindle amplitude remained significant and formed a right lateral area of significance consisting of T4, P4 and C4. Another isolated correlation between CPM scores and fast spindle amplitude on T3 was also significant (Fig. 1 and 2, lower panels). No other sleep spindle parameter was significantly associated with Raven CPM scores after correcting for the effects of age.
For both areas of significance, we directly compared the correlation coefficients of the male and female subsample at the derivation where the correlation was maximal (T4 for slow spindles, P4 for fast spindles) using the Fisher r-to-z method. In both cases, the correlation coefficients are significantly different (zslow=2.88 , pslow=0.004; zfast=2.01 , pfast=0.044), confirming the sexual dimorphism of the spindle amplitude-CPM score association.
Tables 3-5 report the correlations in detail.
Table 3.
Table 4.
Table 5.
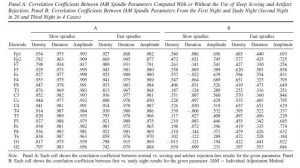
In order to replicate our findings, we also analyzed first-night recordings. This data was of insufficient quality for three children (two female and one male). One further male subject was excluded because for him only a first-night recording was available (for the first night Nmale=11 and Nfemale=13). For another two male subjects, data from C4 and Fp1, respectively, was excluded due to contamination with artifacts. Since a very high correlation was found between IAM spindle parameters calculated from study night (second or third night, see Methods) data with or without using sleep scoring or artifact rejection (see Table 6), after rejecting poor quality subject or channel data we analyzed first-night recordings without sleep stage scoring or further artifact rejection. Spindle parameters showed a modest correlation between the two nights (see Table 6), but the main findings were in general successfully replicated. CPM score correlated with slow spindle amplitude on T4 only in females, both without (rmale=0.315 , pmale=0.345; rfemale=0.574, pfemale=0.04) or with age correction (rmale=-0.08 , pmale=0.826; rfemale=0.681, pfemale=0.015). Without age correction, CPM score also correlated with fast spindle amplitude on P4 only in females (rmale=0.429 , pmale=0.187; rfemale=0.695, pfemale=0.008). However, no significant association was seen after age-correction (rmale=0.185 , pmale=0.609; rfemale=0.475, pfemale=0.119). Neither slow nor fast spindle amplitude correlated with CPM scores in males, with or without age correction. However, both slow and fast spindle density in males (but not females) was positively associated with CPM scores in the first night, an effect which was completely absent in the second night. The associations with fast spindle amplitude in females (with a left and a right lateral area) and slow spindle duration in males (over the entire scalp except for Fp1) remained significant after correcting for multiple comparisons. Age-corrected correlations in the first night are reported in Supplementary Tables 1 and 2. The scalp topography of the association between spindle density, amplitude and CPM scores is illustrated in Supplementary Figures 1 and 2.
Table 6.
Overall, while first night results must be treated with care due to the even lower sample size and generally lower quality of these recordings, the results are consistent with the second night (despite a modest similarity of the spindle parameters themselves) and in favor of a trait-like association between IQ and spindle amplitude in female but not male children.
Discussion
Our study – in line with a previous one in an adolescent sample (Bódizs, et al., 2014) – explicitly aimed to investigate the relationship between CPM performance and sleep spindles using both developmental and trait approaches, that is, specifically investigating whether a correlation between sleep spindle parameters and cognitive performance is due to the effect of age. It also specifically investigated sex differences in the sleep spindle correlates of intelligence at an early stage of development.
Overall, sleep spindles parameters were found to vary little as a function of age at this stage of development, and age effects are not sexually dimorphic, similar to our previous results in adolescents (Bódizs et al. 2014). An increase in fast spindle amplitude seems to be a general
tendency, but it is statistically significant only in a small parietal area where such spindles are prominently present.
The age-uncorrected associations between sleep spindles and Raven CPM scores (corresponding to a developmental approach) also involve both slow and fast spindle amplitude and they are statistically significant only in females. Due to the weak effect of age on sleep spindles, most of the confounding variance in the CPM-spindle relationship comes from the drastic increase of CPM scores with age. Controlling for the effects of age the trait-like associations between intelligence and spindles are revealed. These trait associations between sleep spindles and IQ reveal the same striking sexual dimorphism as previously seen in adolescent (Bódizs, et al., 2014) and adult samples (P. P. Ujma, et al., 2014). Trait IQ appears to be associated with sleep spindles (specifically sleep spindle amplitude, both in case of slow and fast spindles) only in females.
In sum, while age effects on sleep spindling are similar in males and females and involve fast spindle amplitude, even the modest association between CPM scores and fast spindle amplitude in males is virtually completely accounted for by age, suggesting a developmental rather than trait association in male children. In contrast, much like in our previous study in adolescents (Bódizs, et al., 2014), the IQ-spindle amplitude relationship in females is only partially explained by age and in case of slow spindles this association is even stronger if the effects of age are controlled. The fact that this sexually dimorphic association is also present in first-night recordings provides even further support for a trait-like association exclusive for females. While an a correlation between spindle density and CPM scores was found in males in the first night, this was absent in the second night, suggesting a setting-specific effect possibly attributable to better sleep quality or increased sleep-dependent cognitive processing in a novel environment.
It is notable that – unlike in our previous studies in adults and adolescents, which did not reveal any association with slow spindles – both slow and fast spindle amplitude correlated with intelligence. While this seems to be in line with a previous study (Hoedlmoser, et al., 2014), the slow spindle frequency range reported in that paper (11-13 Hz) is rather consistent with the fast spindle range of our study. The fact that both slow and fast spindle amplitude was associated with trait intelligence in female children (and in fact slow spindle effects were more widespread and greater in effect size) suggests that slow spindles may have a more important functional role specifically at this age. However, the precise contribution of slow and fast spindles to cognition across development requires further detailed research.
It is notable that the area of significant age-corrected correlations between CPM and spindle amplitude did not overlap with the scalp midline, where sleep spindles are generally more prominent, but the association was maximal at lateral, especially right lateral derivations for both slow and fast spindles. Given the essentially local nature of spindles (Nir et al., 2011) as well as functional differences between regional spindles (Nishida & Walker, 2007), this result may suggest a functional connection between CPM scores and thalamocortical efficiency in temporal and parietal areas, possibly reflecting more efficient spindle function in more intelligent children in the brain areas responsible for higher order sensory processing, memory and language function which cognitive domains undergo significant improvements over the early years of childhood. This interpretation is, however, admittedly speculative, especially since CPM is a nonverbal intelligence test. Further studies are necessary to assess the importance of local sleep spindle activity and intelligence or specific cognitive abilities in children or adolescents.
Why might sleep spindle amplitude be related to intelligence? The amplitude of sleep spindles depends strongly on the structural characteristics of white matter tracts in adults (Piantoni et al., 2013), which is unsurprising given that these oscillations involve large and spatially separated thalamic and cortical generators. Furthermore, a sexual dimorphism in neuroanatomical correlates of IQ was also reported, with white matter density strongly involved in intelligence of adult female, but less so in male subjects (Gur et al., 1999; Haier, Jung, Yeo, Head, & Alkire, 2005). This pattern of results suggests that (among others) thalamocortical white matter connectivity, reflected by larger spindles, is an important supporting mechanism of intelligence in females, but not males. The presence of this relationship in very young children suggests that sex differences of the neural correlates of IQ may not be only the result of pubertal hormone effects or later psychosocial development. Indeed these differences might emerge at the early phases of the sexual differentiation of the human brain (Alexander & Wilcox, 2012).
Our results show a limited agreement with previous child studies (Chatburn, et al., 2013; Geiger, et al., 2011; Gruber, et al., 2013; Hoedlmoser, et al., 2014). While we did find positive (age-corrected) correlations between spindle amplitude and IQ – similarly to previous results about individually adjusted sigma power (Geiger, et al., 2011) – we found this to be present only in female children. We were unable to replicate the most frequent finding, a negative correlation between spindle frequency and IQ which was found in all previous studies. It is unlikely that this difference is due to the use of different measures of IQ: these studies used either WISC or the Stanford-Binet test to assess intelligence, but they usually reported measures of fluid (as opposed to verbal) intelligence as correlates of sleep spindles. The CPM test we used is a nonverbal, performance-oriented test, which approximates fluid IQ and the g-factor (Prokosch, Yeo, & Miller, 2005; Unsworth & Engle, 2005). A much larger methodological difference is, however, the lack of separate analyses of slow and fast spindles in all but one (Chatburn, et al., 2013) and the lack of individual sleep spindle frequencies in three (Chatburn, et al., 2013; Gruber, et al., 2013; Hoedlmoser, et al., 2014) of the studies. One study (Hoedlmoser, et al., 2014) did not analyze fast spindles because no spectral peaks were detectable in the conventional (13-15 Hz) fast spindle frequency range. Our study, in line with other recent results (McClain et al., 2016) found that sleep spindles in children are rather slow: while clear spectral peaks were visible, all but five subjects had empirically determined slow spindle middle frequencies below the 11 Hz cutoff frequency used by both previous fixed frequency studies, and all subjects had fast spindle middle frequencies below the 13 Hz cutoff point used by the only study (Chatburn, et al., 2013) that used separate slow and fast spindles. These discrepancies make a direct comparison of results – especially those about spindle frequency – especially difficult.
Overall, our study reveals that in case of male children sleep spindles mainly reflect age-related (developmental) changes in intelligence (and even this association is very modest at best). In contrast, sleep spindles are rather a trait-like (age-unrelated) marker of IQ in female children. Increased sleep spindle amplitude in more intelligent female children is in line with previous findings suggesting a sexual dimorphism of the neural correlates of intelligence and a possible triangular relationship between white matter density, sleep spindle amplitude and intelligence in females.
Our study, however, has some significant limitations. First, the size of our sample is quite small due to the difficulty of recruiting young children for a full-night PSG study. This reduces the statistical power of our analyses, potentially overestimating association strengths or missing small but significant relationships. Second, since the parents were allowed to wake up their children after 8.5 hours of sleep, our results are unable to reliably account for the duration-mediated effects of sleep on intelligence. Third, there are differences between the associations reported here and in our previous study about the sleep spindle correlates of intelligence in adults (P. P. Ujma, et al., 2014). Central fast spindle amplitude was associated with IQ in adult females (as opposed to both slow and fast spindle amplitude with a right lateral maximum in female children) and occipital fast spindle density in adult males (as opposed to virtually no significant association in male children). These differences are likely to be of importance, but based on the available data we cannot explain them in detail. Further studies should in any case recruit a greater number of participants (even at the cost of combining multiple samples), especially when the research concerns children.
Acknowledgements
The work was supported by the 2010 Research Grant of the BIAL Foundation (55/10), the Hungarian National Scientific Research Fund (OTKA-K105367) and the Hungarian Brain Research Program (KTIA_NAP_13-1-2013-0001).
References
Abt, K. (1987). Descriptive data analysis: a concept between confirmatory and exploratory data analysis. Methods of Information in Medicine, 26(2), 77-88.
Alexander, G. M.., & Wilcox, T.. (2012). Sex Differences in Early Infancy. Child Development Perspectives, 6(4), 400-406. doi: 10.1111/j.1750-8606.2012.00247.x
Ambrosius, U., Lietzenmaier, S., Wehrle, R., Wichniak, A., Kalus, S., Winkelmann, J., . . . Friess, E. (2008). Heritability of sleep electroencephalogram. Biological Psychiatry, 64(4), 344-348.
Amzica, F., & Steriade, M. (2000). Integration of low-frequency sleep oscillations in corticothalamic networks. Acta Neurobiologiae Experimentalis (Wars), 60(2), 229-245.
Anderson, M.. (2004). Marrying Intelligence and Cognition: A Developmental View. Cognition and Intelligence: Cambridge University Press.
Andrillon, T., Nir, Y., Staba, R. J., Ferrarelli, F., Cirelli, C., Tononi, G., & Fried, I. (2011). Sleep spindles in humans: insights from intracranial EEG and unit recordings. Journal of Neuroscience, 31(49), 17821-17834.
Avants, B. B., Duda, J. T., Kilroy, E., Krasileva, K., Jann, K., Kandel, B. T., . . . Wang, D., J., J. (2015). The pediatric template of brain perfusion. [Data Descriptor]. Scientific Data, 2. doi: 10.1038/sdata.2015.3
Benjamini, Y., & Hochberg, Y.. (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B (Methodological), 289-300.
Bódizs, R., Kis, T., Lazar, A. S., Havran, L., Rigo, P., Clemens, Z., & Halasz, P. (2005). Prediction of general mental ability based on neural oscillation measures of sleep. Journal of Sleep Research, 14(3), 285-292. doi: 10.1111/j.1365-2869.2005.00472.x
Bódizs, R., Gombos, F., Ujma, P. P., & Kovács, I. (2014). Sleep spindling and fluid intelligence across adolescent development: sex matters. [Original Research]. Frontiers in Human Neuroscience, 8. doi: 10.3389/fnhum.2014.00952
Bódizs, R., Körmendi, J., Rigó, P., & Lázár, A. S.. (2009). The individual adjustment method of sleep spindle analysis: Methodological improvements and roots in the fingerprint paradigm. Journal of Neuroscience Methods, 178(1), 205-213. doi: http://dx.doi.org/10.1016/j.jneumeth.2008.11.006
Busby, K., & Pivik, R. T. (1983). Sleep patterns in children of superior intelligence. Journal of Child Psychology and Psychiatry, 24(4), 587-600.
Chatburn, A., Coussens, S., Lushington, K., Kennedy, D., Baumert, M., & Kohler, M. (2013). Sleep spindle activity and cognitive performance in healthy children. Sleep, 36(2), 237-243. doi: 10.5665/sleep.2380
Clawson, B. C., Durkin, J., & Aton, S. J. (2016). Form and Function of Sleep Spindles across the Lifespan. Neural plasticity, 2016.Clemens, Z., Mölle, M., Erőss, L. Jakus, R., Rásonyi, G., Halász, P., & Born, J.. (2011). Fine-tuned coupling between human parahippocampal ripples and sleep spindles. European Journal of Neuroscience, 33(3), 511-520. doi: 10.1111/j.1460-9568.2010.07505.x
De Gennaro, L., Marzano, C., Fratello, F., Moroni, F., Pellicciari, M. C., Ferlazzo, F., . . . Rossini, P. M. (2008). The electroencephalographic fingerprint of sleep is genetically determined: a twin study. Annals of Neurology, 64(4), 455-460.
De Gennaro, L., Ferrara, M., Vecchio, F., Curcio, G., & Bertini, M.. (2005). An electroencephalographic fingerprint of human sleep. NeuroImage, 26(1), 114-122. doi: http://dx.doi.org/10.1016/j.neuroimage.2005.01.020
Doucette, M. R., Kurth, S., Chevalier, N., Munakata, Y., & LeBourgeois, M. K. (2015). Topography of Slow Sigma Power during Sleep is Associated with Processing Speed in Preschool Children. Brain sciences, 5(4), 494-508.
Duffy, F. H., Jones, K., Bartels, P., Albert, M., McAnulty, G. B., & Als, H. (1990). Quantified neurophysiology with mapping: statistical inference, exploratory and confirmatory data analysis. Brain Topography, 3(1), 3-12.
Feinberg, I. (1974). Some Observations on the Reliability of REM Variables. Psychophysiology, 11(1), 68-72.
Finelli, L. A., Achermann, P., & Borbely, A. A. (2001). Individual ‘fingerprints’ in human sleep EEG topography. Neuropsychopharmacology, 25(5 Suppl), S57-62.
Fogel, S. M., Nader, R., Cote, K. A., & Smith, C. T. (2007). Sleep spindles and learning potential. Behavioral Neuroscience, 121(1), 1-10. doi: 10.1037/0735-7044.121.1.1
Fogel, S. M., & Smith, C. T. (2011). The function of the sleep spindle: A physiological index of intelligence and a mechanism for sleep-dependent memory consolidation. Neuroscience & Biobehavioral Reviews, 35(5), 1154-1165. doi: http://dx.doi.org/10.1016/j.neubiorev.2010.12.003
Gaillard, J.,M., Blois, R. (1981). Spindle Density in Sleep of Normal Subjects. Sleep, 4(4), 385-391.
Geiger, A., Achermann, P., & Jenni, O. G. (2010). Sleep, intelligence and cognition in a developmental context: differentiation between traits and state-dependent aspects. Progress in Brain Research, 185, 167-179.
Geiger, A., Huber, R., Kurth, S., Ringli, M., Jenni, O. G., & Achermann, P. (2011). The sleep EEG as a marker of intellectual ability in school age children. Sleep, 34(2), 181-189.
Genzel, L., Kroes, M. C., Dresler, M., & Battaglia, F. P. (2014). Light sleep versus slow wave sleep in memory consolidation: a question of global versus local processes? Trends in Neuroscience, 37(1), 10-19.
Gibson, K., R., & Petersen, A.,C. (1991). Brain maturation and cognitive development: comparative and cross-cultural perspectives: Transaction Publishers.
Gruber, R., Wise, M., S., Frenette, S., Knäauper, B., Boom, A., Fontil, L., & Carrier, J.. (2013). The association between sleep spindles and IQ in healthy school-age children. International Journal of Psychophysiology, 89(2), 229-240. doi: http://dx.doi.org/10.1016/j.ijpsycho.2013.03.018
Gur, R. C., Turetsky, B. I., Matsui, M., Yan, M., Bilker, W., Hughett, P., & Gur, R. E. (1999). Sex differences in brain gray and white matter in healthy young adults: correlations with cognitive performance. Journal of Neuroscience, 19(10), 4065-4072.
Haier, R. J., Jung, R. E., Yeo, R. A., Head, K., & Alkire, M. T. (2005). The neuroanatomy of general intelligence: sex matters. NeuroImage, 25(1), 320-327.
Hassainia, F., Petit, D., & Montplaisir, J. (1994). Significance probability mapping: the final touch in t-statistic mapping. Brain Topography, 7(1), 3-8.
Hoedlmoser, K., Heib, D. P., Roell, J., Peigneux, P., Sadeh, A., Gruber, G., & Schabus, M. (2014). Slow sleep spindle activity, declarative memory, and general cognitive abilities in children. Sleep, 37(9), 1501-1512.
Huupponen, E., Himanen, S. L., Varri, A., Hasan, J., Lehtokangas, M., & Saarinen, J. (2002). A study on gender and age differences in sleep spindles. Neuropsychobiology, 45(2), 99-105.
Iber, C, Ancoli-Israel, S, Chesson, AL, & Quan, SF. (2007). The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specification (1st ed.). Westchester, IL: American Academy of Sleep Medicine.
Landolt, H. P. (2011). Genetic determination of sleep EEG profiles in healthy humans. Progress in Brain Research, 193, 51-61.
Linkowski, P., Kerkhofs, M., Hauspie, R., Susanne, C., & Mendlewicz, J. (1989). EEG sleep patterns in man: a twin study. Electroencephalography and Clinical Neurophysiology, 73(4), 279-284.
McClain, I. J., Lustenberger, C., Achermann, P., Lassonde, J. M., Kurth, S., & LeBourgeois, M. K. (2016). Developmental Changes in Sleep Spindle Characteristics and Sigma Power across Early Childhood. Neural plasticity,2016.
Miller, D. I., & Halpern, D., F. (2014). The new science of cognitive sex differences. Trends in cognitive sciences, 18(1), 37-45. doi: http://dx.doi.org/10.1016/j.tics.2013.10.011
Miller, E., M. (1994). Intelligence and brain myelination: A hypothesis. Personality and Individual Differences, 17(6), 803-832. doi: http://dx.doi.org/10.1016/0191-8869(94)90049-3
Nir, Y., Staba, R. J., Andrillon, T., Vyazovskiy, V. V., Cirelli, C., Fried, I., & Tononi, G. (2011). Regional slow waves and spindles in human sleep. Neuron, 70(1), 153-169.
Nishida, M., & Walker, M. P. (2007). Daytime naps, motor memory consolidation and regionally specific sleep spindles. PLoS One, 2(4), e341.
Piantoni, G., Poil, S. S., Linkenkaer-Hansen, K., Verweij, I. M., Ramautar, J. R., Van Someren, E. J., & Van Der Werf, Y. D. (2013). Individual differences in white matter diffusion affect sleep oscillations. Journal of Neuroscience, 33(1), 227-233.
Prokosch, M., D., Yeo, R., A., & Miller, G.,F. (2005). Intelligence tests with higher g-loadings show higher correlations with body symmetry: Evidence for a general fitness factor mediated by developmental stability. Intelligence, 33(2), 203-213. doi: http://dx.doi.org/10.1016/j.intell.2004.07.007
Raven, J.. (2000). The Raven’s Progressive Matrices: Change and Stability over Culture and Time. Cognitive Psychology, 41(1), 1-48. doi: http://dx.doi.org/10.1006/cogp.1999.0735
Raven, J., C.,& Court, J.,H. (1962). Coloured progressive matrices: HK Lewis London.
Rosanova, M., & Ulrich, D. (2005). Pattern-specific associative long-term potentiation induced by a sleep spindle-related spike train. Journal of Neuroscience, 25(41), 9398-9405.
Schabus, M., Hodlmoser, K., Gruber, G., Sauter, C., Anderer, P., Klosch, G., . . . Zeitlhofer, J. (2006). Sleep spindle-related activity in the human EEG and its relation to general cognitive and learning abilities. European Journal of Neuroscience, 23(7), 1738-1746. doi: 10.1111/j.1460-9568.2006.04694.x
Schabus, M., Dang-Vu, T., T., Albouy, G., Balteau, E., Boly, M., Carrier, J, . . . Gais, S. (2007). Hemodynamic cerebral correlates of sleep spindles during human non-rapid eye movement sleep. Proceedings of the National Academy of Sciences, 104(32), 13164-13169.
Scholle, S., Zwacka, G., & Scholle, H. C. (2007). Sleep spindle evolution from infancy to adolescence. Clinical Neurophysiology, 118(7), 1525-1531. doi: 10.1016/j.clinph.2007.03.007
Shinomiya, S., Nagata, K., Takahashi, K., & Masumura, T. (1999). Development of Sleep Spindles in Young Children and Adolescents. Clinical EEG and Neuroscience, 30(2), 39-43. doi: 10.1177/155005949903000203
Tucker, A. M., Dinges, D. F., & Van Dongen, H. P. (2007). Trait interindividual differences in the sleep physiology of healthy young adults. Journal of Sleep Research, 16(2), 170-180.
Ujma, P. P., Konrad, B. N., Genzel, L., Bleifuss, A., Simor, P., Potari, A., . . . Dresler, M. (2014). Sleep spindles and intelligence: evidence for a sexual dimorphism. Journal of Neuroscience, 34(49), 16358-16368.
Ujma, P. P.Gombos, F., Genzel, L., Konrad, B., N., Simor, P., Steiger, A., . . . Bódizs, R.. (2015). A comparison of two sleep spindle detection methods based on all night averages: individually adjusted versus fixed frequencies. [Original Research]. Frontiers in Human Neuroscience, 9. doi: 10.3389/fnhum.2015.00052
Unsworth, N., & Engle, R., W. (2005). Working memory capacity and fluid abilities: Examining the correlation between Operation Span and Raven. Intelligence, 33(1), 67-81. doi: http://dx.doi.org/10.1016/j.intell.2004.08.003
Urakami, Y., Ioannides, A., A, & K., G., K. (2012). Sleep spindles—as a biomarker of brain function and plasticity (Vol. 4): chapter.
Werth, E., Achermann, P., Dijk, D. J., & Borbely, A. A. (1997). Spindle frequency activity in the sleep EEG: individual differences and topographic distribution. Electroencephalography and Clinical Neurophysiology, 103(5), 535-542.
Copyright notice: this document is under the copyright of the American Psychological Association (APA).