Journal of Psychosomatic Research 99: pp. 95-104. (2017)
DOI: 10.1016/j.jpsychores.2017.05.019
Katalin Z. Ronai1, Andras Szentkiralyi1,2, Alpar S. Lazar1,3, Zsolt I. Lazar4, Istvan Papp4, Ferenc Gombos5, Rezso Zoller6, Maria E. Czira7, Anett V. Lindner8, Istvan Mucsi1,9, Robert Bodizs1, Miklos Z.Molnar10,11, Marta Novak1,12
1Inst. of Behavioural Sciences, Semmelweis University, Budapest, Hungary
2Inst. of Epidemiology and Social Medicine, University of Muenster, Muenster, Germany
3Faculty of Medicine and Health Sciences, University of East Anglia, Norwich, UK
4Dept. of Physics, Babes-Bolyai University, Cluj-Napoca, Romania
5Dept. of General Psychology, Pázmány Péter Catholic University, Budapest, Hungary
61st Dept. of Internal Medicine, Semmelweis University, Budapest, Hungary
7Inst. of Epidemiology and Social Medicine, University of Muenster, Muenster, Germany
8Klinikum Landkreis Erding, Interdisciplinary Pain Center, Erding, Germany
9Dept. of Medicine, Division of Nephrology, University Health Network, University of Toronto, Toronto, Canada
10Dept. Transplantation and Surgery, Semmelweis University, Budapest, Hungary
11Division of Nephrology, Department of Medicine, University of Tennessee Health Science Center, TN, USA
12Centre for Mental Health, University Health Network and Dept. of Psychiatry, University of Toronto, Toronto, Canada
ABSTRACT
Objective
Insomnia complaints are frequent among kidney transplant (kTx) recipients and are associated with fatigue, depression, lower quality of life and increased morbidity. However, it is not known if subjective insomnia symptoms are associated with objective parameters of sleep architecture. Thus, we analyze the association between sleep macrostructure and EEG activity versus insomnia symptoms among kTx recipients.
Methods
Participants (n1 = 100) were selected from prevalent adult transplant recipients (n0 = 1214) followed at a single institution. Insomnia symptoms were assessed by the Athens Insomnia Scale (AIS) and standard overnight polysomnography was performed. In a subgroup of patients (n2 = 56) sleep microstructure was also analyzed with power spectral analysis.
Results
In univariable analysis AIS score was not associated with sleep macrostructure parameters (sleep latency, total sleep time, slow wave sleep, wake after sleep onset), nor with NREM and REM beta or delta activity in sleep microstructure. In multivariable analysis after controlling for covariables AIS score was independently associated with the proportion of slow wave sleep (β = 0.263; CI: 0.026–0.500) and REM beta activity (β = 0.323; CI = 0.041–0.606) (p < 0.05 for both associations).
Conclusions
Among kTx recipients the severity of insomnia symptoms is independently associated with higher proportion of slow wave sleep and increased beta activity during REM sleep but not with other parameters sleep architecture. The results suggest a potential compensatory sleep protective mechanism and a sign of REM sleep instability associated with insomnia symptoms among this population.
Keywords: Beta activity, insomnia, kidney transplant recipients, polysomnography, sleep architecture, slow wave sleep
1.Introduction
Several studies suggest that 50–80% of patients with end-stage kidney disease (ESKD) may have sleep-related problems, including insomnia [1-4], restless legs syndrome [5-7], periodic limb movements in sleep [2, 5-7],and obstructive sleep apnea [8-10]. Successful kidney transplantation might alleviate some sleep problems [3,11], but the prevalence of poor sleep remains remarkably high among these patients: 52.5% among kidney transplant (kTx) recipients were poor sleeper in a cohort study [12]. We previously showed the percentage of kTx recipients who had at least one insomnia complaint was nearly 1.5 times higher compared to the general population [3].
Insomnia and poor sleep are frequent complaints in kTx recipients and they are associated with fatigue [13], depression [3,4], pain [4], post-traumatic stress-symptoms [4] and lower quality of life [3,12,14]. Surprisingly, there is almost a complete lack of information regarding objectively measured sleep parameters in kTx recipients. Altough there are a few published articles that report polysomnographic assessment of sleep in kTx recipients [15-19], most reports focus on obstructive sleep apnea and do not analyze sleep structure in details [16-19].
Insomnia disorder is characterized by difficulty falling asleep, difficulty staying asleep or poor sleep quality, and it leads to impaired daytime functioning, tiredness, fatigue and sleepiness according to the International Classification of Sleep Disorders III and Diagnostic and Statistical Manual of Mental Disorders V criteria [20,21]. These complaints can be of multi-factorial origin among kTx recipients. Anxiety, fear of rejection, deteriorating graft function, altered metabolism of sleep-regulatory mediators, ongoing subclinical inflammation, the presence of other comorbid conditions, immunosuppressant (IS) or other medications and hospitalization may all influence a preexisting sleep disorder or contribute to de novo emergence of sleep problems [22,23].
Diagnosis of insomnia in CKD is similar to the diagnosis among the general population and it is based on clinical interview [23]. The assessment of polysomnography (PSG) is required only when a comorbid sleep-disorder is suspected. However, studies using PSG and detailed EEG analysis contributed important information about the pathophysiology of insomnia among non-kidney disease patients and helped to develop better therapies to improve the subjective symptoms. Patients with insomnia disorder often have longer sleep onset latency (SOL) [24], [25], lower total sleep time (TST) [24-27], dysregulated sleep homeostasis (less slow wave sleep [SWS] or delta activity) [24,26,28,29], or higher wake time after sleep onset (WASO) [24,25,27,30]. Moreover, increased wake-like (beta) EEG activity during sleep is also characteristic for patients with insomnia [27,31,32].
The association of subjective insomnia complaints with objectively assessed sleep architecture and EEG activity (microstructure) have not been investigated before among kTx recipients. However, gaining insight into the objective structure of sleep behind the subjective symptoms might help to treat patients with insomnia more properly among this patients population. Thus, in this study we hypothesized that, similarly to patients with insomnia but no kidney disease, insomnia symptoms are associated with altered sleep macro- and microstructure paramateres (longer SOL, shorter TST, less SWS and delta activity, higher WASO and beta activity).
2.Methods
2.1.Sample of patients and data collection
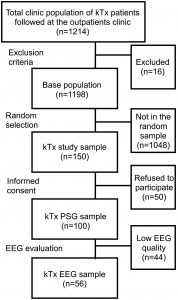
Data for this analysis are obtained from the “SLeep disorders Evaluation in Patients after kidney Transplantation (SLEPT) study” [15,19,33-40]. Potentially eligible patients were selected from all prevalent adult transplant recipients (“total clinic population”; n = 1214) who were regularly followed at a single outpatient academic transplant center, the kidney transplant clinic of the Dept. of Transplantation and Surgery at Semmelweis University, Budapest, Hungary (Fig. 1).
Fig. 1. Selection of patients.
EEG: electroencephalography; kTx: kidney transplant; PSG: polysomnography.
All patients followed at the clinic on December 31, 2006 were considered for enrollment in the Malnutrition and inflammation in transplant (MINIT-HU) study. After applying exclusion criteria (transplant received within < 3 months, presence of active and acute respiratory disorder, acute infection or hospitalization within 1 month, surgery within 3 months), 1198 patients remained (“base population”). From this “base population” we randomly selected and approached 150 patients (“kTx study sample”) using the simple random sampling strategy offered by SPSS 15.0 (IBM Corporation, Armonk, New York, USA).
From these 150 eligible patients (“kTx study sample”), 50 individuals (33%) refused to participate. Consequently, the “kTx PSG sample” who underwent PSG included 100 kTx patients (Fig. 1). There were no significant differences regarding age and sex between the “kTx PSG sample” and those who refused to participate (data not shown). The basic characteristics (age, sex, eGFR, hemoglobin, serum albumin) of the “kTx PSG sample” were similar to the characteristics of the “total clinic population” (data not shown).
Of all PSG recordings in the “kTx PSG sample” 56 had sufficient quality to allow sleep microstruture analysis (“kTx EEG sample”) (Fig. 1). There were no significant differences in the baseline characteristics between the “kTx EEG sample” and the 44 participants excluded from the EEG analysis, except for significantly lower serum albumin in the “kTx EEG sample” (Table A.1).
2.2. Assessment of insomnia
The Athens Insomnia Scale (AIS) was used to assess sleep complaints and to identify possible cases of insomnia [41,42]. The AIS consists of eight items, with score range 0–24, with higher scores indicating worse sleep. Subjects are asked to grade the severity of the sleep complaints (absent, mild, severe, very severe) only if the particular complaint occurred at least three times per week during the last month. A cut-off score of 10 has been suggested for epidemiological studies to detect clinically significant insomnia [42]. The English version of the AIS had been previously translated to Hungarian and validated by our group [43].
2.3. Polysomnography (PSG) and sleep staging
Standard, attended overnight PSG was performed in acoustically isolated and video-monitored sleep laboratory equipped with individual suits (SOMNOscreenTM PSG Tele, SOMNOmedics GmBH, Germany, 0494CE). The following data were recorded: 5 EEG channels (A1, A2, C3, C4, Cz), electrooculogram, chin electromyography, tibial electromyography, electrocardiography, airflow, thoracic–abdominal movements, pulse oximetry, tracheal sound (snoring) and body position. The ground and common reference electrodes were placed at Fpz and Cz, respectively. EEG signals were sampled and stored at 128 Hz, low- and high-pass filters were set at 35 Hz and 0.2 Hz, respectively. All recordings were performed on weekdays, the timing of “lights off” and “lights on” were mostly set around 22:00 and 6:00, respectively.
Recordings were manually scored by two somnologists (MZM, ASL). Sleep stages were determined in 30 s epochs according to Rechtschaffen and Kales [44]. Sleep macroarchitecture was characterized by the following variables: sleep onset latency (SOL: time elapsed from “lights off” to the first occurrence of sleep stage 2); total sleep time (TST); wake after sleep onset (WASO: time spent awake from sleep onset to “lights on”); sleep efficiency (SE: ratio of total sleep time over the time spent in bed); percentages of stages 1, 2, slow wave sleep (SWS: stages 3 and 4 combined) and percentage and latency of REM (rapid eye movement) sleep. (With an illustrative aim we included a hypnogram [Fig. 2.] and a table [Table A.2] summarizing the sleep structure variables measured in the study).
Fig. 2. Hypnogram of a study participant (male, 36 years) to illustrate stages of sleep during the sleep study.
Time is plotted on the abscissa and stages on the ordinate, stages are abbreviated as follows: W: stage wake; R: stage REM; 1: stage 1; 2: stage 2; 3: stage 3; 4: stage 4.
Respiratory events and periodic leg movements were also scored according to standard criteria [45,46]. Apnea was defined as the absence of airflow for > 10 s; hypopnea was defined as a clearly discernible reduction in airflow for > 10 s associated with an arousal and/or 3% reduction in oxygen saturation. The apnea-hypopnea index (AHI) was defined as the number of apneas and hypopneas per hour of sleep. Periodic limb movements were defined as limb movements with duration of 0.5–5 s; inter-movement interval of 5–90 s; and separation criteria for limb movements occurring in both legs: > 5 s between onsets. The periodic limb movement index (PLMI) was defined as the number of limb movements per hours during sleep.
2.4. Analysis of sleep microstructure
Sleep microstructure was analyzed with power spectral analysis. Prior to power spectral analysis EEG artefacts were removed from all EEG channels. For this purpose, EEG segments containing artefacts were visually identified and annotated on a 4-s basis by an experienced somnologist using our custom-made software (FerciosEEG, © Ferenc Gombos 2008–2016) [47]. EEG signals were subsequently exported, while the annotated segments were excluded from further analysis. Power spectral density averaged over the whole night was calculated separately for NREM (stages 2, 3 and 4) and REM sleep stages.
Artefact-free EEG segments of interest were concatenated and power density was calculated for central derivations (C3-Cz, C4-Cz) using Welch’s periodogram method as averages over detrended Hanning windowed 4-s long epochs with 50% overlap. Frequency-specific absolute spectral powers (in μV2/Hz) were obtained as the integral of the cubic spline interpolated power values over the delta (0.75–4 Hz), theta (4–8 Hz), alpha (8–11 Hz), sigma (11–15 Hz) and beta (15–25 Hz) bands divided by the width of the respective band [48-50] (With an illustrative aim we included an EEG power spectrum picture (Fig. 3) of a patient and we summerized the different frequency bands in Table A.2). The algorithms were based on the NumPy, SciPy, and Matplotlib libraries for scientific computing [51,52].
Fig. 3. EEG power spectrump of a patient (female, 25 years) during NREM and REM sleep.
The power spectrum is a function that represents the strength (power) of EEG oscillations at each studied frequency bin.
2.5. Assessment of depression and comorbidities
We assessed depressive symptoms with the Hungarian version of the Center for Epidemiologic Studies – Depression (CES-D) scale [53]. This was translated and validated by our team using standard procedures [54]. This questionnaire contains 20 items that ask participants to grade how frequently their complaints occured (rarely, 1–2 days, 3–4 days, 5–7 days) within the last week. The total CES-D score was used to describe psychological distress in the sample.
Comorbidity was assessed by the modified Charlson Comorbidity Index [55] completed by the main responsible transplant physician of the participant. Information about medication use was obtained from the questionnaires and the medical charts.
2.6. Laboratory data
Laboratory data were extracted from the medical charts, including blood hemoglobin, serum albumin and creatinine. Estimated glomerular filtration rate (eGFR) was calculated using the “4-variable” CKD-EPI (Chronic Kidney Disease EPIdemiology collaboration) formula [56]. All laboratory data was within a month of the sleep study.
2.7. Transplantation and donor related data
Transplantation-related information collected included current medications, transplant and dialysis “vintage” (i.e., time elapsed since transplantation or time spent on dialysis prior to transplantation), history of acute rejection, age and sex of donor and history of delayed graft function. Time elapsed since the initiation of the first treatment for ESKD – cumulative ESKD time – was also calculated. Standard maintenance IS therapy generally consisted of prednisolone, either cyclosporine A microemulsion formulation or tacrolimus, combined with mycophenolate mofetil or azathioprine, everolimus or sirolimus. All enrolled kTx recipients were receiving maintenance IS therapy during our study.
2.8. Ethics approval
The study was approved by the Research Ethics Board of the Semmelweis University (4/2007). Before enrollment, patients received detailed verbal and written information about the aims and protocol of the study and signed an informed consent.
2.9. Statistical analysis
Statistical analysis was carried out using STATA 13.0 software. Continuous variables were compared using Student’s t-test or the Mann-Whitney U test, as appropriate. Categorical variables were analyzed using the chi-square test or Fisher’s exact test where the observation numbers were low. Correlation analysis was performed using Pearson and Spearman rank correlation analysis.
We analyzed the association between the AIS score and the PSG macrostructure parameters and frequency power spectra with multivariable linear regression. The models were built with the sleep parameter as dependent variable and the AIS score as independent variable. At first step we included the AIS score, age, sex and graft function into Model 1. Additionally the CES-D score and hypnotic medication use variables were also included into Model 2. We selected the covariables based on theoretical considerations.
We used transformations (square root – SOL; logarithmic – WASO, power spectra) to achieve normal distribution of the variables where it was necessary. In all statistics, two-sided tests were used and p < 0.05 was considered statistically significant.
We also performed multivariable analyses of sleep macro- and microstructure versus AIS score including additional covariables (AHI, PLMI, Charlson Comorbidity Index, IS medications – data not shown) and these variables did not alter the results considerably. Thus, we decided not to include these analyses in the present report.
3.Results
The demographic and laboratory parameters, comorbid conditions and transplantation related data of the “kTx PSG sample” are presented in Table 1. Descriptive data of sleep macrostructure parameters are presented in Table 2.
Table 1. Baseline characteristics of the kTx PSG sample and the subgroups Insomnia and Non-insomnia.
Table 1. Baseline characteristics of the kTx PSG sample and the subgroups Insomnia and Non-insomnia.
|
kTx PSG sample |
Insomnia (AIS ≥ 10) |
Non-insomnia (AIS < 10) |
p |
|
|
n (%) |
100 (100) |
16 (16) |
84 (84) |
N/A |
|
Age (mean ± SD) (years) |
51 ± 13 |
57 ± 12 |
50 ± 13 |
0.024 |
|
Male (%) |
57 |
50 |
58 |
0.537 |
|
BMI (mean ± SD) (kg/m2) |
27 ± 5 |
28 ± 6 |
27 ± 5 |
0.167 |
|
eGFR (mean ± SD) (ml/min/1.73 m2) |
54 ± 19 |
46 ± 14 |
55 ± 20 |
0.042 |
|
Serum albumin (mean ± SD) (g/L) |
40 ± 3 |
40 ± 3 |
40 ± 4 |
0.412 |
|
Charlson comorbidity index (median;IQR) |
2;1 |
3;2 |
2;1 |
0.035 |
|
Prevalence of diabetes (%) |
19 |
31 |
17 |
0.179 |
|
Prevalence of hypertension (%) |
92 |
94 |
92 |
1.000 |
|
Prevalence of insomnia (%) (AIS 10 cut-off) |
16 |
100 |
0 |
N/A |
|
AIS (median; IQR) |
4;6 |
12;5 |
3;5 |
< 0.001 |
|
Prevalence of depression (%) (CES-D 18 cut-off) |
21 |
44 |
16 |
0.012 |
|
CES-D (median; IQR) |
9;11 |
17;18 |
9;9 |
0.035 |
|
Hypnotic drug use (%) |
17 |
37 |
13 |
0.017 |
|
Antipdepressant use (%) |
2 |
6 |
1 |
0.296 |
|
Transplant vintage (median; IQR) (months) |
66;83 |
72;71 |
76;85 |
0.642 |
|
IS medication (%) |
||||
|
Steroid |
85 |
81 |
86 |
0.704 |
|
Cyclosporine |
43 |
56 |
40 |
0.243 |
|
Azathiophrine |
5 |
6 |
5 |
1.000 |
|
Sirolimus |
12 |
0 |
14 |
0.205 |
|
Mycophenolate-mofetil |
71 |
75 |
70 |
1.000 |
|
Tacrolimus |
46 |
38 |
48 |
0.587 |
|
Dialysis vintage (median; IQR) (months) |
18;32 |
26;32 |
18;30 |
0.225 |
|
Cumulative ESKD time (median; IQR) (months) |
101;91 |
107;78 |
101;94 |
0.694 |
Insomnia was defined by the cut-off score of the Athens Insomnia Scale (AIS < 10: non-insomnia subgroup; AIS ≥ 10: insomnia subgroup). AIS: Athens Insomnia Scale; BMI: body mass index; CES-D: Center for Epidemiologic Studies – Depression scale; eGFR: estimated glomerular filtrate rate; ESKD: end-stage kidney disease; Immunosuppressant: IS; IQR: interquartile range; kTx: kidney transplant; SD: standard deviation.
Table 2. Polysomnography parameters of the kTx PSG sample and their correlations with AIS score.
|
kTx PSG sample |
AIS score Spearman’s rho |
p |
|
|
SOL (median;IQR) (min) |
15;17 |
0.105 |
0.301 |
|
SE (median;IQR) (%) |
80;13 |
− 0.179 |
0.075 |
|
TST (mean ± SD) (h) |
6 ± 1.3 |
− 0.127 |
0.214 |
|
Stage 1 (mean ± SD) (%) |
11 ± 6 |
− 0.053 |
0.599 |
|
Stage 2 (mean ± SD) (%) |
43 ± 13 |
− 0.020 |
0.844 |
|
SWS (mean ± SD) (%) |
12 ± 8 |
0.060 |
0.557 |
|
REM sleep (mean ± SD) (%) |
13 ± 6 |
− 0.159 |
0.115 |
|
REM latency (median;IQR) (min) |
145;87 |
0.142 |
0.165 |
|
WASO (median;IQR) (min) |
61;47 |
0.088 |
0.392 |
|
AHI (median; IQR) (1/h) |
4;14 |
− 0.026 |
0.798 |
|
PLMI (median; IQR) (1/h) |
6;15 |
− 0.094 |
0.354 |
|
|
|
|
|
AHI: apnea-hypopnea index; AIS: Athens Insomnia Scale; kTx: kidney transplant; PLMI: periodic limb movement index; REM: rapid eye movement; SE: sleep efficiency; SOL: sleep onset latency, SWS: slow wave sleep; TST: total sleep time; WASO: wake after sleep onset.
3.1. Prevalence and severity of insomnia symptoms and their associations with descriptive and sleep architecture parameters
The median; IQR AIS score was 4; 6 in the “kTx PSG sample” (Table 1). Sixteen percent of the kTx recipients had high risk of insomnia based on the AIS cut-off score. Seventeen percent of the patients were using hypnotic medication.
High risk of insomnia was significantly associated with older age, worse kidney function (lower estimated glomerular filtration rate [eGFR]), higher number of comorbidities, higher prevalence of hypnotic drug use and presence of depression and severity of depressive symptoms (Table 1). These associations were analyzed and discussed in more details in our previous publication about a larger sample of kTx recipients (n = 884) [3].
High risk for insomnia was not associated with sleep macrostructure parameters in our sample (Table A.3). However, there was a trend towards a negative correlation between the AIS score and SE (r = − 0.178; p = 0.075) (Table 2). No other associations were present between the AIS score and sleep macrostructure.
3.2. Multivariable analysis of insomnia symptoms versus sleep macrostructure
We analyzed the selected sleep macrostructure parameters with multivariable linear regression (Table 3). After controlling for potential confounders (Model 1: age, sex, eGFR and Model 2: age, sex, eGFR, CES-D, hypnotic medication use) higher AIS score was significantly associated with higher proportion of SWS (β: 0.263; CI: 0.026–0.500; p = 0.030). AIS score was not associated with SOL, TST or WASO in our sample. (With an explorative aim we also analyzed other sleep parameters in multivariable analysis. The results are presented in Table A.4).
Table 3. Associations of sleep macrostructure parameters (dependent variable) and AIS score (independent variable) in multivariable linear regression models (n = 100).
Table 3. Associations of sleep macrostructure parameters (dependent variable) and AIS score (independent variable) in multivariable linear regression models (n = 100).
|
Dependent variable |
β coefficient |
95% CI |
p |
|
|
SOL (min) |
Model 1 |
− 0.023 |
− 0.179–0.225 |
0.824 |
|
Model 2 |
− 0.048 |
− 0.285–0.188 |
0.686 |
|
|
TST (h) |
Model 1 |
− 0.133 |
− 0.349–0.082 |
0.223 |
|
Model 2 |
− 0.120 |
− 0.374–0.134 |
0.350 |
|
|
SWS (%) |
Model 1 |
0.113 |
− 0.095–0.322 |
0.282 |
|
Model 2 |
0.263 |
0.026–0.500 |
0.030 |
|
|
WASO (min) |
Model 1 |
0.046 |
− 0.158–0.250 |
0.656 |
|
Model 2 |
0.088 |
− 0.158–0.335 |
0.478 |
|
Model 1: adjusted for: AIS score, age, sex, eGFR.
Model 2: adjusted for: AIS score, age, sex, eGFR, CES-D score, hypnotic medication use.
AIS: Athens Insomnia Scale; CES-D: Center for Epidemiologic Studies – Depression scale; CI: confidence interval; eGFR: estimated glomerular filtrate rate; SOL: sleep onset latency, SWS: slow wave sleep; TST: total sleep time; WASO: wake after sleep onset.
3.3. Associations of insomnia symptoms and sleep EEG spectra
In the next step we analyzed the association between AIS score and absolute power spectra (Table 4). The AIS score significantly correlated with the REM sigma frequency band (r = 0.287; p = 0.032) and there were trends towards a positive correlation with REM beta (r = 0.257; p = 0.055) and NREM alpha (r = 0.244; p = 0.070). The AIS score was not associated with power spectra of NREM beta, NREM delta or REM delta in the univariable analysis.
Table 4. Descriptive data of sleep EEG spectra and correlations of AIS score versus sleep EEG spectra of the kTx EEG sample (n = 56).
|
Spectrum absolute power μV2/Hz (median; IQR) |
AIS score Spearman’s rho |
p |
|
|
NREM |
|||
|
Beta |
0.30;0.23 |
0.194 |
0.152 |
|
Sigma |
2.5;3.2 |
0.142 |
0.300 |
|
Alpha |
3.3;3.4 |
0.244 |
0.070 |
|
Theta |
5.4;3.5 |
0.151 |
0.266 |
|
Delta |
51.5;37 |
0.012 |
0.931 |
|
REM |
|||
|
Beta |
0.32;0.25 |
0.257 |
0.055 |
|
Sigma |
0.68;0.58 |
0.287 |
0.032 |
|
Alpha |
1.4;1.2 |
0.211 |
0.118 |
|
Theta |
3.2;2.4 |
0.083 |
0.545 |
|
Delta |
14;9.2 |
0.197 |
0.145 |
AIS: Athens Insomnia Scale; IQR: interquartile range; kTx: kidney transplant; NREM: non-rapid eye movement sleep; EEG: electroencephalography; REM: rapid eye movement sleep.
We further analyzed the association of beta and delta power spectra with the AIS score in multivariable regression models (Table 5). After adjusting for covariables the AIS score was independently associated with higher REM beta (Model 2, β: 0.323; CI: 0.041–0.606; p = 0.026). Power spectra of NREM beta and delta or REM delta were not associated with AIS score (Table 5). (With an explorative aim we also analyzed other frequency bands in multivariable analysis. The results are presented in Table A.5.)
Table 5. Associations of sleep EEG spectra (dependent variable) and AIS score (independent variable) in multivariable linear regression models (n = 56).
|
Dependent variable |
β coefficient |
95% CI |
p |
|
|
NREM sigma |
Model 1 |
0.177 |
− 0.052–0.406 |
0.127 |
|
Model 2 |
0.045 |
− 0.206–0.297 |
0.718 |
|
|
NREM alpha |
Model 1 |
0.279 |
0.016–0.541 |
0.038 |
|
Model 2 |
0.256 |
− 0.050–0.563 |
0.099 |
|
|
NREM theta |
Model 1 |
0.117 |
− 0.142–0.377 |
0.367 |
|
Model 2 |
0.192 |
− 0.107–0.491 |
0.202 |
|
|
REM sigma |
Model 1 |
0.345 |
0.075–0.614 |
0.013 |
|
Model 2 |
0.306 |
− 0.005–0.616 |
0.054 |
|
|
REM alpha |
Model 1 |
0.259 |
− 0.031–0.550 |
0.079 |
|
Model 2 |
0.325 |
− 0.012–0.663 |
0.058 |
|
|
REM theta |
Model 1 |
0.132 |
− 0.160–0.423 |
0.369 |
|
Model 2 |
0.255 |
− 0.077–0.586 |
0.129 |
|
Model 1: adjusted for: AIS score, age, sex, eGFR.
Model 2: adjusted for: AIS score, age, sex, eGFR, CES-D score, hypnotic medication use.
AIS: Athens Insomnia Scale; CES-D: Center for Epidemiologic Studies – Depression scale; CI: confidence interval; EEG: electroencephalography; eGFR: estimated glomerular filtrate rate; NREM: non-rapid eye movement sleep; REM: rapid eye movement.
4.Discussion
This is the first study analyzing detailed sleep architecture and sleep EEG activity associated with insomnia symptoms in kTx recipients. Our main finding is that the severity of insomnia symptoms is associated with higher amount of SWS and higher beta power during REM sleep.
While there is a growing literature about the importance of subjective sleep quality among kTx recipients [3,4,12,57], only limited information has been published about the objectively measured sleep characteristics in this population. Three prospective studies have reported on the change of sleep disordered breathing before and after kidney transplantation [16-18]. These studies yielded inconsistent results regarding the improvement of sleep architecture following kidney transplantation and reported incomplete data of sleep macrostructure parameters. In the studies which reported SOL [16,17], TST [16], SWS [16-18], and WASO [17] the values were comparable with our findings. Additionally, in our previous report we presented data about the severity of depressive symptoms and its associations with sleep macrostructure among kTx recipients [15].
To gain insight to the sleep characteristics of kTx recipients associated with insomnia symptoms first we analyzed sleep macrostructure. Contrary to our expectations, univariable analysis did not reveal any significant associations between insomnia symptoms and sleep macrostructure. Similarly, SOL, TST and WASO parameters were not associated with symptoms of insomnia in multivariable analysis, either. Similar negative findings were also reported in patients with different medical conditions comorbid with insomnia [58].
Although the previously mentioned parameters of sleep macrostructure were not associated with symptoms of insomnia in the current study, contrary to our expectations, higher proportion of SWS was associated with higher AIS score in the fully adjusted multivariable model. In the general population insomnia has been characterized by lower homeostatic sleep pressure as indicated by less SWS [24,28]. Our results, however, suggest an increased homeostatic sleep pressure associated with higher subjective insomnia complaints in kTx patients. This may be either a homeostatic rebound/compensation to a less restorative sleep quality experienced on a chronic basis by the patients or may be due to intrinsic circadian factors, subclinical inflammation and/or medication. Unfortunately, our study design does not enable us to answer this question and further studies are needed to determine if an increased proportion of SWS is related to the treatment modality itself or to other biological factors related to the kidney disease.
The so-called “paradoxical” increase of SWS was also described in patients with chronic fatigue syndrome [59,60], and this might reflect an attempt to compensate an impaired process reflected in ultraslow oscillations [60,61]. Besides insomnia symptoms, prevalence of fatigue is also very high among ESKD and in a study worse subjective sleep quality and higher proportion of stage 2 sleep (suggesting higher sleep spindling – sleep protection of NREM sleep) was associated with higher fatigue score [62]. SWS was not different between the high fatigue versus low fatigue groups, however, associations of fatigue with sleep macrostructure parameters were not analyzed in multivariable analysis.
Proportion of SWS varies among patients with different stages of CKD and different treatment modalities for ESKD. In a large study of > 1740 participants with intact or various degree of impaired renal function, patients with more advanced CKD tended to have shorter SWS duration after adjustment for age, sex, sleep disordered breathing severity and PLMI [63]. In another work ESKD patients treated with ambulatory peritoneal dialysis (APD) had higher proportion of SWS (after adjustment for age, sex, race and body mass index) compared to patients with earlier stages of CKD, despite that APD patients had worse subjective sleep quality [64]. In two prospective PSG studies the proportion of stage 3 sleep was significantly higher after kTx compared to hemodialysis [17,18], however, in one study SWS significantly decreased after kTx [16].
In sleep microstructure analysis we could not confirm the association of higher homeostatic sleep pressure with insomnia symptoms: NREM delta power was not associated with AIS in our sample. However, this might be due to methodological reasons. Here we analyzed NREM stages 2,3 and 4 combined and not SWS separately. Furthermore, we had a 5 channel EEG, however, it is known that differences in the power spectrum is not only present at central sites. According to literature additional recording for example at frontal sites might rather be informative to capture localized association of insomnia symptoms and delta EEG activity [25,26].
In this analysis we also hypothetized that higher wake-like EEG activity (beta) is associated with more severe insomnia symptoms among this population. Interestingly, we did not observe an association between AIS score and beta frequency band during NREM sleep, however, during REM sleep higher beta activity was independently associated with insomnia symptoms after adjustment for covariables. Increased beta power during REM sleep was also found in the work of Merica et al. who investigated primary insomnia subjects [26].
A growing interest has recently been directed to assess the association between “REM instability” and insomnia [65]. According to this theory microarousals or wake intrusions preceding or during REM sleep or relative cortical activation during REM could contribute to altered time-perception [25,30,66]. This, in turn, might worsen the perception of sleep and lead to sleep-maintenance insomnia complaints. Interestingly, sleep-maintenance insomnia based on the AIS score was found to be the most frequent subtype of insomnia among kTx recipients in our previous work [3]. Additionally, we found that prolonged REM latency and less REM sleep were associated with the severity of insomnia symptoms after controlling for age, sex and graft function in multivariable models (data was presented in the Appendix A). After additional adjustment for depressive symptoms and hypnotic medication use these associations did not remain significant, however, this might be due to the relatively small sample size and consequent low statistical power of our study. These results together with the association of REM beta activity and insomnia symptoms may indicate higher instability of REM sleep among kTx recipients with more severe symptoms.
Several limitations of this study should be considered when interpreting our results. First of all, admittedly, our study sample was quite heterogeneous. Importantly, we did not exclude patients with obstructive sleep apnea or periodic limb movement disorder from our analysis. Although in studies of insomnia disorder the presence of these sleep disorders usually represent exclusion criteria, sleep disorders in kTx recipients in most cases are present simultaneously and excluding those patients would have largely limited enrollment. Furthermore, we wanted to study sleep characteristics in a “real life” clinical patient population on a sample which is representative to “the total clinic population”. For these reasons we decided to select the patients with random sampling and considered obstructive sleep apnea or periodic limb movement disorder as potential confounders. (We adjusted the models for AHI and PLMI as well and these variables did not alter the results considerably – data was not shown).
Second, we assessed only a one-night PSG, and for this reason first-night effect might have influenced our findings. We did not offer a two-night assessment to enhance acceptability and participation in the study. However, the lack of an adaptation night might be one of the reasons why the EEG recordings contained substantial amount of artefact.
Third, patients in this study were taking multiple medications and this might influenced our results. We have very little specific knowledge about the effect of IS treatment on EEG spectra and on sleep structure. As we mentioned in Methods, we also performed the analysis including IS medications as additional covariables in the multivariable models, and they did not alter the results of this report. Additionally, seventeen percent of the patients were taking sleeping pills that can modify EEG activity during sleep [67,68]. For this reason we statistically controlled our models for hypnotic medication use.
Lastly, we analyzed sleep microstructure only in a subgroup of patients of the “kTx PSG sample”. We excluded 44 EEG-s prior to power spectral analysis based on the quality of the EEG recordings. This might have introduced selection bias since patients with worse sleep may “produce” lower quality sleep recordings (patients excluded from the power spectral analysis did have significantly lower SE and shorter TST – data was not shown). However, clinical and socio-demographic characteristics of excluded subjects were comparable to those remaining in the final “kTx EEG sample” (Table A.1), suggesting that the results are still representative to the “total clinic population”.
In summary, in our present report insomnia symptom severity was associated with higher proportion of SWS and higher REM beta power in the fully adjusted models. (Other parameters of sleep macrostructure and beta and delta power during NREM sleep were not associated with insomnia symptoms among kTx recipients.) The observed alterations might be signs of an impaired homeostatic sleep regulation and instability of REM sleep associated with insomnia symptoms among this population.
This study is the first analysis that reports sleep architecture in a relatively high number of kTx recipients and its association with insomnia symptoms. Most importantly we would like to draw attention to the significance of sleep complaints among this population and highlight that insomnia symptoms are associated with different alterations in sleep architecture than in the non-kidney disease population. Other works are needed to replicate our findings and to explore in more details whether higher homeostatic sleep pressure associated with insomnia symptoms is due to some yet undefined factors (p.e: fatigue, treatment modality, medications, subclinical inflammation). Future research should also investigate whether appropriate interventions to improve sleep (such as sleep hygiene, encouraging siesta, relaxation before sleep or cognitive behavioural therapy for insomnia, CBT-i) would change these observed sleep parameters and also improve subjective sleep complaints of kTx recipients.
Part of this manuscript has been accepted as poster for the 5th annual conference of the European Association of Psychosomatic Medicine.
Acknowledgements
The authors thank patients and staff of the Dept. of Transplantation and Surgery and the Sleep Laboratory at the 1st Dept. of Internal Medicine, Semmelweis University, Budapest, Hungary. The analysis was performed at the Inst. of Behavioural Sciences, Semmelweis University, Budapest, Hungary. RB received personal fees from Pharma Nord Hungary – unrelated to this research. MZM received a Grant from NIH and had a position at Merck Advisory Board – both were unrelated to this research. The other authors have indicated no financial conflicts of interest.
Appendix A.
Table A.1. Descriptive data of the kTx EEG sample (n = 56) and excluded patients (n = 44).
|
kTx EEG sample |
Excluded patients |
p |
|
|
n |
56 |
44 |
N/A |
|
Age (mean ± SD) (years) |
49 ± 13 |
53 ± 12 |
0.068 |
|
Male (%) |
62.5 |
50 |
0.210 |
|
BMI (mean ± SD) (kg/m2) |
26 ± 4 |
27 ± 5 |
0.165 |
|
eGFR (mean ± SD) (ml/min/1.73 m2) |
52 ± 18 |
55 ± 21 |
0.218 |
|
Serum albumin (mean ± SD) (g/L) |
40 ± 4 |
41 ± 3 |
0.042 |
|
Charlson comorbidity index (median;IQR) |
2;1 |
2;1 |
0.585 |
|
Prevalence of diabetes (%) |
23 |
14 |
0.226 |
|
Prevalence of hypertension (%) |
89 |
95 |
0.460 |
|
Prevalence of insomnia (%) (AIS 10 cut-off) |
18 |
14 |
0.568 |
|
AIS (median; IQR) |
4;8 |
3.5;4 |
0.735 |
|
Prevalence of depression (%) (CES-D 18 cut-off) |
25 |
14 |
0.178 |
|
CES-D (median; IQR) |
10;12 |
9;9 |
0.260 |
|
Hypnotic drug usage (%) |
23 |
9 |
0.106 |
|
Antidepressant usage (%) |
2 |
2 |
1.000 |
|
Transplant vintage (median; IQR) (months) |
63;78 |
77;84.5 |
0.718 |
|
IS medication (%) |
|||
|
Steroid |
86 |
84 |
0.821 |
|
Cyclosporine |
46 |
39 |
0.435 |
|
Azathiophrine |
2 |
9 |
0.166 |
|
Sirolimus |
12.5 |
11 |
1.000 |
|
Mycophenolate-mofetil |
70 |
73 |
0.736 |
|
Tacrolimus |
43 |
50 |
0.477 |
|
Everolim |
0 |
2 |
0.440 |
|
Dialysis vintage (median; IQR) (months) |
22;40.5 |
15.5;23 |
0.354 |
|
Cumulative ESKD time (median; IQR) (months) |
101;95 |
100.5;91 |
0.957 |
AIS: Athens Insomnia Scale; BMI: body mass index; CES-D: Center for Epidemiologic Studies – Depression scale; eGFR: estimated glomerular filtrate rate; ESKD: end-stage kidney disease; Immunosuppressant: IS; IQR: interquartile range; kTx: kidney transplant; SD: standard deviation.
Table A.2. Sleep macro- and microstructure parameters.
|
Sleep macrostructure parameters |
Definition |
Abbreviation |
|
Sleep onset latency |
Time elapsed from “lights off” to the first occurrence of sleep stage 2 |
SOL |
|
Sleep efficiency |
Ratio of total sleep time over the time spent in bed |
SE |
|
Total sleep time |
Time spent asleep |
TST |
|
Percentage of stage 1 sleep |
Percentage of stage 1 sleep within sleep period time (time from sleep onset until final awakening) |
Stage 1 |
|
Percentage of stage 2 sleep |
Proportion of stage 2 sleep within sleep period time |
Stage 2 |
|
Slow wave sleep |
Proportion of stages 3 and 4 combined within sleep period time |
SWS |
|
REM latency |
Time elapsed from sleep onset to the first occurence of REM sleep |
– |
|
REM percentage |
Proportion of REM sleep within sleep period time |
REM |
|
Spectral bands |
Description |
Frequency range |
|
Delta |
Typical activity during SWS |
0.75–4 Hz |
|
Theta |
Typical activity during stage 1 and 2 sleep and REM sleep |
4–8 Hz |
|
Alpha |
Typical activity during resting wakefulness |
8–11 Hz |
|
Sigma |
Activity of sleep spindles |
11–15 Hz |
|
Beta |
Typical activity during alert wakefulness |
15–25 Hz |
Table A.3. Polysomnography parameters of the kTx PSG sample and subgroups Insomnia (n = 16) and Non-insomnia (n = 84).
|
kTx PSG sample |
Insomnia (AIS ≥ 10) |
Non-insomnia (AIS < 10) |
p |
|
|
SOL (median;IQR) (min) |
15;17 |
16;21 |
15;18 |
0.527 |
|
SE (median;IQR) (%) |
80;13 |
75;12 |
81;13 |
0.114 |
|
TST (mean ± SD) (h) |
6 ± 1.3 |
5.9 ± 1 |
6.1 ± 1.4 |
0.282 |
|
Stage 1 (mean ± SD) (%) |
11 ± 6 |
10 ± 6 |
11 ± 7 |
0.652 |
|
Stage 2 (mean ± SD) (%) |
43 ± 13 |
41 ± 13 |
43 ± 13 |
0.297 |
|
SWS (mean ± SD) (%) |
12 ± 8 |
13 ± 7 |
12 ± 8 |
0.253 |
|
REM sleep (mean ± SD) (%) |
13 ± 6 |
11 ± 6 |
13 ± 6 |
0.126 |
|
REM latency (median;IQR) (min) |
145;87 |
175;121 |
145;76 |
0.341 |
|
WASO (median;IQR) (min) |
61;47 |
74;53 |
59;47 |
0.175 |
|
AHI (median; IQR) (1/h) |
4;14 |
2;6 |
4;21 |
0.384 |
|
AHI < 5: without OSA (%) |
57 |
69 |
55 |
0.411 |
|
AHI ≥ 30: severe OSA (%) |
14 |
6 |
15 |
0.458 |
|
PLMI (median; IQR) (1/h) |
6;15 |
3;21 |
7;15 |
0.821 |
|
PLMI < 5: without PLMD (%) |
48 |
56 |
46 |
0.471 |
|
PLMI ≥ 25: severe PLMD (%) |
16 |
19 |
15 |
0.717 |
Insomnia was defined by the cut-off score of the Athens Insomnia Scale (AIS < 10: non-insomnia subgroup; AIS ≥ 10: insomnia subgroup). AHI: apnea-hypopnea index; AIS: Athens Insomnia Scale; IQR: interquartile range; kTx: kidney transplant; REM: rapid eye movement; OSA: obstructive sleep apnea; PLMD: periodic limb movement disorder; PLMI: periodic limb movement index; SD: standard deviation; SE: sleep efficiency; SWS: slow wave sleep; SOL: sleep onset latency, TST: total sleep time; WASO: wake after sleep onset.
Table A.4. Associations of sleep macrostructure parameters (dependent variable) and the AIS score (independent variable) in multivariable linear regression models (n = 100).
|
Dependent variable |
β coefficient |
95% CI |
p |
|
|
SE (%) |
Model 1 |
− 0.144 |
− 0.341–0.052 |
0.149 |
|
Model 2 |
− 0.120 |
− 0.350–0.111 |
0.306 |
|
|
Stage 1 (%) |
Model 1 |
− 0.062 |
− 0.260–0.137 |
0.538 |
|
Model 2 |
− 0.082 |
− 0.321–0.157 |
0.498 |
|
|
Stage 2 (%) |
Model 1 |
− 0.067 |
− 0.285–0.150 |
0.540 |
|
Model 2 |
− 0.190 |
− 0.437–0.058 |
0.131 |
|
|
REM sleep (%) |
Model 1 |
− 0.217 |
− 0.415– -0.018 |
0.033 |
|
Model 2 |
− 0.117 |
− 0.353–0.120 |
0.328 |
|
|
REM latency (min) |
Model 1 |
0.234 |
0.027–0.442 |
0.027 |
|
Model 2 |
0.235 |
− 0.008–0.478 |
0.057 |
|
Model 1: adjusted for: AIS score, age, sex, eGFR.
Model 2: adjusted for: AIS score, age, sex, eGFR, CES-D score, hypnotic medication use.
We used transformations to achieve normal distribution of the variables when it was necessary (SE: cubic, stage 1 sleep and REM latency: square transformation).
AIS: Athens Insomnia Scale; CES-D: Center for Epidemiologic Studies – Depression scale; CI: confidence interval; eGFR: estimated glomerular filtrate rate; REM: rapid eye movement; SE: sleep efficiency.
Table A.5. Associations of sleep EEG spectra (dependent variable) and AIS score (independent variable) in multivariable linear regression models (n = 56).
|
Dependent variable |
β coefficient |
95% CI |
p |
|
|
NREM sigma |
Model 1 |
0.177 |
− 0.052–0.406 |
0.127 |
|
Model 2 |
0.045 |
− 0.206–0.297 |
0.718 |
|
|
NREM alpha |
Model 1 |
0.279 |
0.016–0.541 |
0.038 |
|
Model 2 |
0.256 |
− 0.050–0.563 |
0.099 |
|
|
NREM theta |
Model 1 |
0.117 |
− 0.142–0.377 |
0.367 |
|
Model 2 |
0.192 |
− 0.107–0.491 |
0.202 |
|
|
REM sigma |
Model 1 |
0.345 |
0.075–0.614 |
0.013 |
|
Model 2 |
0.306 |
− 0.005–0.616 |
0.054 |
|
|
REM alpha |
Model 1 |
0.259 |
− 0.031–0.550 |
0.079 |
|
Model 2 |
0.325 |
− 0.012–0.663 |
0.058 |
|
|
REM theta |
Model 1 |
0.132 |
− 0.160–0.423 |
0.369 |
|
Model 2 |
0.255 |
− 0.077–0.586 |
0.129 |
|
Model 1: adjusted for: AIS score, age, sex, eGFR.
Model 2: adjusted for: AIS score, age, sex, eGFR, CES-D score, hypnotic medication use.
We used transformations to achieve normal distribution of the variables when it was necessary (power spectra: logarithmic transformation).
AIS: Athens Insomnia Scale; CES-D: Center for Epidemiologic Studies – Depression scale; CI: confidence interval; EEG: electroencephalography; eGFR: estimated glomerular filtrate rate; NREM: non-rapid eye movement sleep; REM: rapid eye movement.
References
[1] E.A. Iliescu, K.E. Yeates, D.C. HollandQuality of sleep in patients with chronic kidney disease
Nephrol. Dial. Transplant., 19 (1) (2004), pp. 95-99
[2] I. Mucsi, et al.Sleep disorders and illness intrusiveness in patients on chronic dialysis
Nephrol. Dial. Transplant., 19 (7) (2004), pp. 1815-1822
[3] M. Novak, et al.Chronic insomnia in kidney transplant recipients, Am. J. Kidney Dis., 47 (4) (2006), pp. 655-665
[4] P.G. Liaveri, et al.Quality of sleep in renal transplant recipients and patients on hemodialysis, Psychosom. Res., 93 (2017), pp. 96-101
[5] M.L. Unruh, et al. Restless legs symptoms among incident dialysis patients: association with lower quality of life and shorter survival, Am. J. Kidney Dis., 43 (5) (2004), pp. 900-909
[6] G.L. Gigli, et al. Restless legs syndrome in end-stage renal disease, Sleep Med., 5 (3) (2004), pp. 309-315
[7] M.Z. Molnar, et al. Restless legs syndrome in patients after renal transplantation, Am. J. Kidney Dis., 45 (2) (2005), pp. 388-396
[8] W.C. Chen, et al.Sleep behavior disorders in a large cohort of Chinese (Taiwanese) patients maintained by long-term hemodialysis, Am. J. Kidney Dis., 48 (2) (2006), pp. 277-284
[9] C.J. de Oliveira Rodrigues, et al.Relationship among end-stage renal disease, hypertension, and sleep apnea in nondiabetic dialysis patients, Am. J. Hypertens., 18 (2 Pt 1) (2005), pp. 152-157
[10] M.Z. Molnar, et al.High prevalence of patients with a high risk for obstructive sleep apnoea syndrome after kidney transplantation–association with declining renal function, Nephrol. Dial. Transplant., 22 (9) (2007), pp. 2686-2692
[11] M. Russcher, et al.The effects of kidney transplantation on sleep, melatonin, circadian rhythm and quality of life in kidney transplant recipients and living donors, Nephron, 129 (1) (2015), pp. 6-15
[12] M. Sabbatini, et al.Sleep quality in renal transplant patients: a never investigated problem, Nephrol. Dial. Transplant., 20 (1) (2005), pp. 194-198
[13] J.R. Rodrigue, et al.A cross-sectional study of fatigue and sleep quality before and after kidney transplantation, Clin. Transpl., 25 (1) (2011), pp. E13-E21
[14] M. Sabbatini, et al.Renal transplantation and sleep: a new life is not enough, Nephrol., 21 (Suppl. 13) (2008), pp. S97-101
[15] K.Z. Ronai, et al. Depressive symptoms are associated with objectively measured sleep parameters in kidney transplant recipients,Clin. Sleep Med., 13 (4) (2017), pp. 557-564
[16] J.M. Beecroft, et al. Impact of kidney transplantation on sleep apnoea in patients with end-stage renal disease, Nephrol. Dial. Transplant., 22 (10) (2007), pp. 3028-3033
[17] B. Jurado-Gamez, et al. Kidney transplantation improves sleep-related breathing in hemodialysis patients, Blood Purif., 26 (6) (2008), pp. 485-490
[18] C.J. Rodrigues, et al. Sleep-disordered breathing changes after kidney transplantation: a polysomnographic study, Nephrol. Dial. Transplant., 25 (6) (2010), pp. 2011-2015
[19] K. Fornadi, et al. Sleep apnea is not associated with worse outcomes in kidney transplant recipients, Sci. Rep., 4 (2014), p. 6987
[20] American Academy of Sleep Medicine International Classification of Sleep Disorders: Diagnostic and Coding Manual (third ed.), American Academy of Sleep Medicine, Darien, IL (2014)
[21] American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders, (fifth ed.), American Psychiatric Association, Arlington, VA (2013)
[22] M.Z. Molnar, M. Novak, I. Mucsi Sleep disorders and quality of life in renal transplant recipients, Int. Urol. Nephrol., 41 (2) (2009), pp. 373-382
[23] A.V. Lindner, et al.Insomnia in patients with chronic kidney disease, Semin. Nephrol., 35 (4) (2015), pp. 359-372
[24] L. Huang, et al.Polysomnographically determined sleep and body mass index in patients with insomnia, Psychiatry Res., 209 (3) (2013), pp. 540-544
[25] G. St-Jean, et al.REM and NREM power spectral analysis on two consecutive nights in psychophysiological and paradoxical insomnia sufferers, Int. J. Psychophysiol., 89 (2) (2013), pp. 181-194
[26] H. Merica, R. Blois, J.M. GaillardSpectral characteristics of sleep EEG in chronic insomnia, Eur. J. Neurosci., 10 (5) (1998), pp. 1826-1834
[27] K. Spiegelhalder, et al. Increased EEG sigma and beta power during NREM sleep in primary insomnia, Biol. Psychol., 91 (3) (2012), pp. 329-333
[28] W.R. Pigeon, M.L. PerlisSleep homeostasis in primary insomnia, Sleep Med. Rev., 10 (4) (2006), pp. 247-254
[29] A.D. Krystal, et al. NREM sleep EEG frequency spectral correlates of sleep complaints in primary insomnia subtypes, Sleep, 25 (6) (2002), pp. 630-640
[30] A.D. Perusse, et al. REM sleep as a potential indicator of hyperarousal in psychophysiological and paradoxical insomnia sufferers, Int. J. Psychophysiol., 95 (3) (2015), pp. 372-378
[31] D. Riemann, et al. The hyperarousal model of insomnia: a review of the concept and its evidence, Sleep Med. Rev., 14 (1) (2010), pp. 19-31
[32] J.C. Levenson, D.B. Kay, D.J. Buysse The pathophysiology of insomnia, Chest, 147 (4) (2015), pp. 1179-1192
[33] M.Z. Molnar, et al. Sleep apnea is associated with cardiovascular risk factors among kidney transplant patients, Clin. J. Am. Soc. Nephrol., 5 (1) (2010), pp. 125-132
[34] M.Z. Molnar, et al.Association between the malnutrition-inflammation score and post-transplant anaemia, Nephrol. Dial. Transplant., 26 (6) (2011), pp. 2000-2006
[35] M.Z. Molnar, et al.Evaluation of the malnutrition-inflammation score in kidney transplant recipients, Am. J. Kidney Dis., 56 (1) (2010), pp. 102-111
[36] C.P. Kovesdy, et al. Body mass index, waist circumference and mortality in kidney transplant recipients, Am. J. Transplant., 10 (12) (2010), pp. 2644-2651
[37] C.P. Kovesdy, et al. Associations between serum leptin level and bone turnover in kidney transplant recipients, Clin. J. Am. Soc. Nephrol., 5 (12) (2010), pp. 2297-2304
[38] C.P. Kovesdy, et al. Association of serum phosphorus level with anemia in kidney transplant recipients, Transplantation, 91 (8) (2011), pp. 875-882
[39] M.Z. Molnar, et al. Association of the malnutrition-inflammation score with clinical outcomes in kidney transplant recipients, Am. J. Kidney Dis., 58 (1) (2011), pp. 101-108
[40] K. Fornadi, et al. Lack of association between objectively assessed sleep disorders and inflammatory markers among kidney transplant recipients, Int. Urol. Nephrol., 44 (2) (2012), pp. 607-617
[41] C.R. Soldatos, D.G. Dikeos, T.J. PaparrigopoulosAthens insomnia scale: validation of an instrument based on ICD-10 criteria, Psychosom. Res., 48 (6) (2000), pp. 555-560
[42] C.R. Soldatos, D.G. Dikeos, T.J. Paparrigopoulos The diagnostic validity of the Athens insomnia scale, Psychosom. Res., 55 (3) (2003), pp. 263-267
[43] M. Novak, et al. Increased utilization of health services by insomniacs–an epidemiological perspective, Psychosom. Res., 56 (5) (2004), pp. 527-536
[44] A.K.A. Rechtschaffen A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. Brain Information Service/Brain Research Institute, University of California, Los Angeles (1968)
[45] Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The report of an American academy of sleep medicine task force. Sleep, 1999. 22(5): p. 667–689.
[46] A.S. Walters, et al. The scoring of movements in sleep, Clin. Sleep Med., 3 (2) (2007), pp. 155-167
[47] A. Kis, et al. Development of a non-invasive polysomnography technique for dogs (Canis familiaris), Physiol. Behav., 130 (2014), pp. 149-156
[48] A.S. Lazar, et al.Reduced fronto-cortical brain connectivity during NREM sleep in Asperger syndrome: an EEG spectral and phase coherence study, Clin. Neurophysiol., 121 (11) (2010), pp. 1844-1854
[49] A.S. Lazar, Z.I. Lazar, D.J. Dijk Circadian regulation of slow waves in human sleep: topographical aspects, NeuroImage, 116 (2015), pp. 123-134
[50] A.S. Lazar, et al. Sleep deficits but no metabolic deficits in premanifest Huntington’s disease, Ann. Neurol., 78 (4) (2015), pp. 630-648
[51] J.D. Hunter Matplotlib: a 2D graphics environment, Comput. Sci. Eng., 9 (2007), pp. 90-95
[52] T.E. Oliphant Python for scientific computing, Comput. Sci. Eng., 9 (2007), pp. 10-20
[53] L. Radloff The CES-D Scale — a self-report depression scale for research in the general population, Appl. Psychol. Meas., 1 (1977), pp. 385-401
[54] D.E. Beaton, et al. Guidelines for the process of cross-cultural adaptation of self-report measures, Spine (Phila Pa 1976), 25 (24) (2000), pp. 3186-3191
[55] S.V. Jassal, D.E. Schaubel, S.S. Fenton Baseline comorbidity in kidney transplant recipients: a comparison of comorbidity indices, Am. J. Kidney Dis., 46 (1) (2005), pp. 136-142
[56] A.S. Levey, et al. A new equation to estimate glomerular filtration rate, Ann. Intern. Med., 150 (9) (2009), pp. 604-612
[57] J.M. Williams, et al. A novel application of a biopsychosocial theory in the understanding of disturbed sleep before and after kidney transplantation, Clin. Sleep Med., 12 (2) (2015), pp. 247-256
[58] R. Budhiraja, et al. Prevalence and polysomnographic correlates of insomnia comorbid with medical disorders, Sleep, 34 (7) (2011), pp. 859-867
[59] D. Neu, et al. High slow-wave sleep and low-light sleep: chronic fatigue syndrome is not likely to be a primary sleep disorder, Clin. Neurophysiol., 26 (3) (2009), pp. 207-212
[60] O. Le Bon, et al. Ultra-slow delta power in chronic fatigue syndrome, Psychiatry Res., 200 (2–3) (2012), pp. 742-747
[61] D. Neu, et al.Slow wave sleep in the chronically fatigued: power spectra distribution patterns in chronic fatigue syndrome and primary insomnia, Clin. Neurophysiol., 126 (10) (2015), pp. 1926-1933
[62] M. Jhamb, et al. Prevalence and correlates of fatigue in chronic kidney disease and end-stage renal disease: are sleep disorders a key to understanding fatigue? Am. J. Nephrol., 38 (6) (2013), pp. 489-495
[63] A. Ogna, et al. Sleep characteristics in early stages of chronic kidney disease in the hypnolaus cohort, Sleep, 39 (4) (2016), pp. 945-953
[64] M.E. Roumelioti, et al. Objective and subjective sleep disorders in automated peritoneal dialysis, Can. J. Kidney Health Dis., 3 (2016), p. 6
[65] D. Riemann, et al.REM sleep instability–a new pathway for insomnia? Pharmacopsychiatry, 45 (5) (2012), pp. 167-176
[66] B. Feige, et al. Does REM sleep contribute to subjective wake time in primary insomnia? A comparison of polysomnographic and subjective sleep in 100 patients, Sleep Res., 17 (2) (2008), pp. 180-190
[67] A.A. Borbely, et al. Effect of benzodiazepine hypnotics on all-night sleep EEG spectra, Hum. Neurobiol., 4 (3) (1985), pp. 189-194
[68] X. Tan, et al. Long-, intermediate- and short-acting benzodiazepine effects on human sleep EEG spectra, Psychiatry Clin. Neurosci., 57 (1) (2003), pp. 97-104
© 2017 Elsevier Inc. All rights reserved.