Purpose
The primary purpose of the Viral Vector Core is to use a Biosafety Level 2 laboratory at the Institute of Translational Medicine (EOK Laboratory 0.112), open to both Semmelweis University and academic working groups, for the production and preclinical use of viral vectors. In recent years, cell reprogramming technologies have revolutionised in vitro techniques and allowed to create and study many human-induced pluripotent stem cells (iPSCs) and directly reprogrammed disease-specific cellular subtypes (transdifferentiated, so-called induced cells). Notwithstanding, the Nobel Prize-winning CRISPR/CAS9 gene editing technology was presented. Together, these combined methodological possibilities have opened up unprecedented research perspectives, mostly in genetic modelling, preclinical investigation and validation of complex human diseases. The new Viral Vector Core Facility provides a unique platform of state-of-the-art technologies for the Semmelweis University and all academic groups.
Background
The usage for in vivo validation of human/mouse/rat 2D or 3D primary cell cultures is a necessity for many clinical trials. Transfection of primary cells, specific tissues, organs is extremely challenging in the lab. Transduction, using viral vectors in in vitro and in vivo experimental models provides a widely used and well-controlled solution. Viral Vector Core Facilities are present at numerous universities to provide in-house viral vector production, which is undoubtedly a huge advantage in basic and applied research activities.
The Viral Vector Core Facility at the Semmelweis University offers a shared organisational unit functioning as a platform. The platform provides researchers viral vectors in a unique way: from designing and cloning to production, and titration. The platform provides research teams with access to state-of-the-art vector technologies, preclinical and other translational research. These experiments will contribute to essential information that can lead to subsequent innovative clinical trials with high patenting potential.
Aims
- Generation of lentiviral (overexpression of cDNA, shRNA, miRNA, etc.)
Design and cloning
Production, titration and quality control (QC) of viral vectors
- Generation of CRISPR/CAS9 lentiviral vectors for gene modification, gene therapy
CRISPR KO, CRISPRi (CRISPR interference), CRISPRa (CRISPR activation)
Design and cloning
Production, titration and quality control (QC) of viral vectors
Workplan
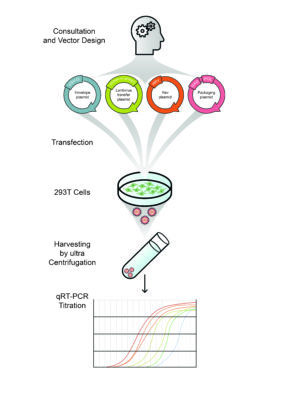
Researchers/clinicians first deliver their plasmid of interest to the platform. It is also possible to design the vector plasmid via consultation. The platform will also help to select the right promoter, CRISPR technology and the most suitable vector design for in vivo or in vitro experiments. The Viral Vector Core Facility will start viral vector production which can take up 4 to 6 weeks (depending on cloning):
- Transfection of the vector plasmid into HEK 293T cells.
- Collection and concentration of the supernatant by ultracentrifugation.
- Titration (qRT-PCR).
- Concentration and batch amount of a typical GFP-containing viral vector is 80-100 ul of 10E9 TU/mL from 2 x T175 cell culture flasks.
Services
Services are available to all internal and external academic customers
(external and international customers please contact us for pricing).
Semmelweis University users have priority access.
All prices INCLUDE TAX.
Cloning
Construct design and cloning strategies consultation
Construct building / full cloning service:
150 000 – 250 000 HUF (depending on cloning strategy)
Construct testing and validation e.g. restriction digestion, sequencing
Maxiprep Service (or other DNA scale-up):
50 000 HUF
Related molecular biology services (tell us about your molecular biology needs)
Lentiviral vector production services
Full service production of lentivirus.
Vectors will be delivered in 5ul/tube or 10ul/tube or 50ul/tube aliquots (depending on selected concentration.
-
- standard batch – high concentration, delivers 70-90ul of 108-109 TUI/ml*
- standard batch – normal concentration, delivers 300ul of 107-108 TUI/ml*
(*depending on plasmid size and titration protocol)
- For Lentiviral vectors please provide the following amounts of donor plasmid DNA: LV-standard 45ug (LV-large 135ug).
- The quality of the DNA matters, please ensure that you have performed restriction digest and/or sequencing and have an OD ratio of 1.7-2.0 and deliver at a concentration of 0.5-1.0ug/ul.
| standard batch 1x | 150 000 HUF |
| standard batch 2x | 300 000 HUF |
| standard batch 3x | 450 000 HUF |
| standard batch 4x | 600 000 HUF |
| standard batch 5x | 750 000 HUF |
| standard batch 6x |
900 000 HUF |
Contact
Ágnes Varga, PhD
Phone: +36 30 016 47 87
E-mail: varga.agnes1@semmelweis.hu
0.112 laboratory room
D part, ground floor
Basic Medical Science Center
Institute of Translational Medicine
Semmelweis University
1094 Budapest, Tűzoltó street. 37–47
This project is supported by: STIA-KFI, TKP-NVA