Ideggyogy Sz 2014; 67(1-2):59-68.
Róbert BÓDIZS1, Ferenc GOMBOS2, Katalin SZÔCS3, János M. RÉTHELYI3, Patrícia GERVÁN2, Ilona KOVÁCS2
1 Semmelweis Egyetem, Magatartástudományi Intézet, Budapest
2 Pázmány Péter Katolikus Egyetem, Általános Lélektani Tanszék, Budapest
3 Semmelweis Egyetem, Pszichiátriai és Pszichoterápiás Klinika, Budapest
Correspondent: Dr. Róbert BÓDIZS, Institute of Behavioural Sciences, Semmelweis University; 1089 Budapest, Nagyvárad tér 4. Phone: (06-1) 210-2953, fax: (06-1) 210-2955. E-mail: bodrob@net.sote.hu
Abstract
Background and purpose – Reports on twin pairs concordant and discordant for Williams syndrome were published before, but no study unravelled sleep physiology in these cases yet. We aim to fill this gap by analyzing sleep records of a twin pair discordant for Williams syndrome extending our focus on presleep wakefulness and sleep spindling.
Methods – We performed multiplex ligation-dependent probe amplification of the 7q11.23 region of a 17 years old dizygotic opposite-sex twin pair discordant for Williams syndrome. Polysomnography of laboratory sleep at this age was analyzed and followed-up after 1.5 years by ambulatory polysomnography. Sleep stages scoring, EEG power spectra and sleep spindle analyses were carried out.
Results – The twin brother showed reduced levels of amplification for all of the probes in the 7q11.23 region indicating a typical deletion spanning at least 1.038 Mb between FKBP6 and CLIP2. The results of the twin sister showed normal copy numbers in the investigated region. Lower sleep times and efficiencies, as well as higher slow wave sleep percents of the twin brother were evident during both recordings. Roughly equal NREM, Stage 2 and REM sleep percents were found. EEG analyses revealed state and derivation-independent decreases in α power, lack of an α spectral peak in presleep wakefulness, as well as higher NREM sleep σ peak frequency in the twin brother. Faster sleep spindles with lower amplitude and shorter duration characterized the records of the twin brother. Spectra show a striking reliability and correspondence between the two situations (laboratory vs. home records).
Conclusion – Alterations in sleep and specific neural oscillations including the α/σ waves are inherent aspects of Williams syndrome.
Absztrakt
Háttér és célok – A Williams-szindrómára concordans és discordans ikrekrôl eddig közölt esettanulmányok adósak maradtak az alvásélettani sajátosságok elemzésével. Célunk ezt a hiányt pótolni egy Williams-szindrómára discordans ikerpár alvásregisztrátumainak elemzésével, miközben vizsgálatunkat az elalvás elôtti ébrenlétre és az alvási orsózás sajátosságaira is kiterjesztjük.
Módszerek – A 17. életévükben járó, kétpetéjû, eltérô nemû és Williams-szindrómára discordans ikrek 7q11.23 kromoszómarégióit multiplex ligációfüggô próba amplifikációs eljárással vizsgáltuk. Az alváslaboratóriumban készült poliszomnográfiás felvételben, valamint a másfél évvel ezután megismételt ambuláns poliszomnográfiás regisztrátumban az alvásstádiumokat, az EEG-teljesítményt és az alvási orsózást elemeztük.
Eredmények – A fiú ikertestvér eredményei valamennyi próbában csökkent amplifikációt mutattak, ami tipikusnak mondható, legalább 1,038 Mb kiterjedésû, az FKBP6 és CLIP2 között elhelyezkedô deléció. A lánytestvér eredményei normál másolatszámokat eredményeztek a vizsgált régióban. A fiútestvér mindkét regisztrátumában alacsonyabb szintû alvásidôt és hatékonyságot, valamint emelkedett lassú hullámú alvási arányt találtunk a lánytestvérhez képest. A NREM, a 2. stádiumú és a REMalvási arány az ikertestvérekben megközelítôleg egyenlô szintû volt. Az EEG-elemzések az α-teljesítmény állapot- és elvezetésfüggetlen csökkenését, az elalvást megelôzô ébrenlétben hiányzó spektrális α-csúcsot, továbbá a NREM-fázisban magasabb spektrális σ csúcsfrekvenciát tártak fel a fiútestvér felvételeiben. Magasabb frekvenciájú, alacsonyabb amplitúdójú és rövidebb idôtartamú alvási orsókat is megfigyeltünk a fiútestvér felvételeiben. A spektrumok figyelemreméltó egyezést mutattak a két helyzet (laboratórium vs. otthon) között.
Következtetések – Az alvásban, valamint a specifikus – α/σ hullámokat érintô – neuralis oszcillációkban bekövetkezô módosulások a Williams-szindróma inherens részét képezik.
Introduction
Sleep architecture and/or sleep EEG spectral parameters were shown to be altered in several developmental disabilities, including autism, Asperger syndrome, Down syndrome, fragile X syndrome and attention deficit hyperactivity disorder (ADHD)1–4. Williams syndrome (WS) is a genetically determined developmental disorder linked to a microdeletion of 25 to 28 genes on chromosome 7q11.23 and characterized by mild to moderate mental retardation, learning difficulties, cardiovascular abnormalities, high sociability and empathy and a distinctive cognitive-linguistic profile5, 6. Perhaps this latter aspect is the most interesting for cognitive research: severe visual-spatial deficits and relative strength in expressive language7, 8. As overactivity and shortened attention span are typical in WS, it is not surprising that more than 50% of WS individuals are diagnosed with ADHD as well9, 10. Although subjective reports suggest a particularly high level of sleeping difficulties in WS, alterations of actigraphic and polygraphic sleep were only scarcely investigated. Previous studies reported difficulties in initiating and maintaining sleep, increased slow wave sleep, decreased REM sleep and periodic leg movements during sleep in those children with WS whose parents reported sleep problems11. Actigraphic studies12 and polysomnographic investigations13, 14 suggest the continuity of most of these problems into the postpubertal ages, however the data is still incomplete and scarce. Additionally, sleep EEG spectra was shown to be characterized by increased frontally-derived delta activity as well as by decreased α and σ power in both NREM and REM sleep13. Last, but not least a special alteration in the spectral profile of the 8–16 Hz NREM sleep EEG of WS subjects was also reported. This altered profile was characterized by decreased α/low σ activity, redistribution of power toward higher σ frequencies as well as higher spectral peak frequencies in the 8–16 Hz range15. As there is unequivocal evidence for the thesis that psychosocial, socioeconomic and environmental factors may substantially influence brain development, neural activity and behaviour16, 17, studies on twin pairs discordant for WS provide a unique opportunity for revealing anatomical, physiological or cognitive alterations specific to this syndrome. Twin pairs concordant and discordant for WS have been reported in the literature. In line with unequal homologous recombination as the cause and the 100% penetrance of this genetic syndrome, monozygotic twins were shown to be concordant, while dizygotic twins discordant for WS18-22. No twin study reported sleep behaviour or physiology in subjects with WS before. Here we report the laboratory and ambulatory polygraphic sleep of an opposite sex twin pair discordant for WS. Our focus is on the macrostructure of sleep and on the EEG profiles of our subjects. We aim to confirm the reproducibility and reliability of the recently reported differences in sleep structure and EEG spectra of subjects with WS compared to the typically developing (TD) ones13, 15 in the twin pair discordant for WS, but sharing socioeconomical and environmental factors with potential developmental and sleep-related relevance. In order to achieve this goal we first present a detailed picture of the sleep laboratory records of the twin pair previously enrolled in the polysomnographic and spectral EEG studies of WS13, 15. Here we pay special attention to previously reported peculiarities in the sleep physiology of WS subjects11, 13, 14 in order to test whether these features are reflected in the differences between the twin with WS and his TD sister. Furthermore, we aim to test the reliability of these findings by repeating polysomnography after a period of 1.5 years in the twins’ home, using an ambulatory recording device. Such tests of reliability are rarely used in the sleep studies of the developmentally disabled population15, but are particularly relevant in discerning the individual sleep EEG profiles in twin studies15, 23. In previous reports we focused on WS- vs. TD NREM and REM sleep EEG band power differences as well as on 8–16 Hz NREM sleep EEG spectral profiles. Neither data on WS-specific EEG activity in wakeful resting conditions, nor a detailed analysis on NREM sleep EEG spindling (spindle densities, durations and amplitudes) was given. In this case study we provide preliminary information on these aspects using the data of the twin pair included in previous studies13, 15.
Case history
There was no previous history of genetic disorders in the family of the twins investigated in this study. The mother was a 33-year-old primipara receiving standard pregnancy care, the twin pregnancy was diagnosed by ultrasonography during the 10th gestational week. The twins were delivered per vias naturales preterm on the 35th weeks after a high risk pregnancy in 1991. Both twins required rehospitalization during the first months of their life due to growth delay and bronchopulmonary infections.
CASE 1
The boy weighed 2.250 grams at birth. Pediatric examination after birth revealed dysmorphic body features and a varus foot, the latter required orthopaedic examination and physiotherapy. Because of the dysmorphic body features he was tested for metabolic disorders, yielding negative results. The chromosome examination indicated normal karyotype. At the age of 13 months the parents experienced abnormal facial twitching and on one occasion tonal-clonal movement of the lower limb. The subsequent electroencephalographic examination showed abnormal epileptic activity, the patient received low dose phenobarbital and primidone medication until the age of six years. He underwent right side orchidectomy because of cryptorchism in 1996. Cardiologic examination in 1998 showed mitral prolapse and grade I. mitral insufficiency, this was controlled later throughout his life, and he received endocarditis prophylactic treatment. As an infant the boy showed slow gross motor development, he started sitting up at 18 months of age, standing at 19 months of age, and walking when he was three. The fine motor development was also delayed and his fine-motor deficits persisted throughout his life (e.g. clumsy grasping). Language development was also atypically slow, he performed only babbling at the age of three years, started to say distinct basic words at 4.5 and to talk fluently at 5.5 years of age. Based on the symptoms of mental retardation, hypermotility, and mitral insufficiency, the diagnosis of WS was established in 1998, by a positive fluorescent in situ hybridization (FISH) test. At the age of 16 years, neuropsychological investigation found mild mental retardation, poor visuo-spatial abilities, visual memory deficits. His language perception and production was sufficient, although he expressed himself in simple sentences. He attended a special primary and secondary school for mentally disabled children. In 2010 abdominal ultrasonography and computer tomography showed a coarctation of the abdominal aorta. Due to the good general status no surgical intervention was necessary, antihypertensive therapy was initiated. He passed away unexpectedly due to sudden death in May 2011, autopsy confirmed cardiωly and acute heart failure.
CASE 2
The sister of the patient weighed 2.350 grams at birth. She showed no sign of developmental disorder or delay during her childhood. She attended normal public schools and performed at a good academic level. At the age of 21 years she is a college student.
Methods
GENETIC ANALYSES
The twins were tested for WS at the age of seven years with FISH of the elastine (ELN) gene as part of the routine clinical genetic diagnostic procedure. To further characterize and specify the extent of copy number variation, i.e. the size of the hemidelition of the affected twin, we performed multiplex ligation-dependent probe amplification (MLPA) using the SALSA MLPA KIT P029-A1 (MRCHolland, Amsterdam, The Netherlands) according to the established protocol24, 25. The MLPA reaction, a robust and reliable method, is based on the hybridization of specific oligonucleotide probes to the sample DNA and the consequential amplification of the hybridized targets after ligation. Amplification fragments can be separated and quantified afterwards using capillary electrophoresis. The P029-A1 assay contains probes for the following genes in the 7q11.2 chromosomal region: FKBP6, FZD9, TBL2, STX1A, exons 1,6,20, and 33 in ELN, LIMK1, RFC2, and two loci in CLIP2. The probe mix also contains 20 reference probes.
LABORATORY POLYSOMNOGRAPHY
Sleep was recorded in 2008 in the laboratory for two consecutive nights, according to the subjects sleeping habits (lights on and lights off at the preferred time of the subjects). Electroencephalograms (EEG) according to the 10-20 system26 at 18 recording sites (Fp1, Fp2, F3, F4, F7, F8, Fz, C3, C4, Cz, P3, P4, T3, T4, T5, T6, O1, O2) referred to the contralateral mastoids, left and right electrooculograms (EOG) with electrodes placed at the left and right outer canthi (contralateral mastoid reference), respectively, as well as bipolar submental electromyograms (EMG), electrocardiograms (ECG), and accelerometry-based left and right leg movement detections were carried out. Gold-coated Ag/AgCl electrodes fixed with EC2 Grass Electrode Cream (Grass Technologies, USA) served for EEG recordings, while EOG, EMG and ECG recordings were performed with disposable electrodes (T40/80, Telic S.A., Spain). Impedances for the EEG electrodes were kept below 5 k. Signals were collected, pre-filtered, amplified and digitized at a sampling rate of 249 Hz/channel by using the 30 channel Flat Style SLEEP La Mont Headbox with implemented second order filters at 0.5 Hz (high pass) and 70 Hz (low pass) as well as the HBX32-SLP 32 channel preamplifier (La Mont Medical Inc. USA). An additional 50 Hz digital notch filtering performed by the DataLab acquisition software (Medcare, Iceland) was carried out before data conversion (European Data Format or EDF)27. EEG channels were re-referenced to the mathematically-linked mastoids before quantitative EEG analyses. Sleep recordings were visually scored according to standard criteria28 in 20 second epochs. The following definitions were used for sleep architecture evaluation: sleep efficiency (calculated as the percent of sleep duration / the time in bed), wake time after sleep onset (WASO), as well as NREM, Stage 1, Stage 2, slow wave sleep (SWS, defined as the amount of time in Stages 3 and 4) and REM sleep percent. The sleep architectural data of the twins was compared with group means (expected values) of WS (N=9) and TD (N=9) subjects (data from ref. 13). Deviations from the confidence intervals (±1.96 SE) were considered as a significant. Next the 4 second epochs containing artefactual sleep EEG were manually removed before further automatic sleep EEG analyses. Average power spectral densities were calculated by a mixed-radix Fast Fourier Transformation (FFT) algorithm applied to the 50 percent overlapping, Hanningtapered, artefact-free 4 second (996 points) epochs of whole night stages 2–4 NREM sleep and REM sleep separately. Moreover, the Individual Adjustment Method (IAM) of sleep spindle analysis29 was used to unravel the potential peculiarities of sleep EEG spindling in WS. In short the principle of sleep spindle detection is the idea that individual spindles are those groups of waves which last at least 0.5 seconds and contribute to one or two of the major peaks in the 9–16 Hz average amplitude spectra of NREM sleep EEG. Individual-specific spectral peaks were formalized by calculating the zero crossing points of their second order derivatives. The EEGs recorded during presleep wakefulness were first subjected to Independent Component Analysis (ICA) in order to reduce the movement artefacts and keep as much records for analysis as possible. After ICA-based artefact filtering a visual decision on further spectral analyses was made on a 4 second basis. Spectral analysis followed the procedure described above.
AMBULATORY POLYSOMNOGRAPHY
On the follow up, after 1.5 years (2009) subjects’ sleep was recorded at their homes by using ambulatory home polysomnography. Sleep recordings on two consecutive weekend nights were performed according to the subjects sleeping habits. We used a portable 32 channel SD LTM Headbox together with a BRAIN QUICK System PLUS software (Micromed, Italy) for polysomnographic data recording. EEG and polygraphic data was high-pass filtered at 0.15 Hz and low-pass filtered at 1500 Hz (both 40 dB/decade). Data were collected with an analogue to digital conversion rate of 4096 Hz/channel (synchronous, 16 bit). A further 40 db/decade anti-aliasing digital filter was applied by digital signal processing (firmware) which low pass filtered the data at 124 Hz. Subsequently, the digitized and filtered EEG was undersampled and stored on a sampling rate of 1024 Hz. The analyses were similar to the ones used in the laboratory sleep study: EDF-converted EEG-signals classified into Rechtschaffen and Kales28 stages (20 s) were broken into 4 second (4096 points) epochs, artefactual ones removed and the remaining ones subjected to FFT. Average spectra of whole night NREM (Stages 2–4) and REM sleep were calculated by averaging the periodograms of Hanning-tapered and 50% overlapping epochs, re-referenced to the mathematically-linked mastoids. Last, but not least we used the IAM of sleep spindle analysis in order to characterize sleep spindling in the ambulatory records. Presleep wakefulness was cleaned from artefacts by removing critical ICA-components, then visually screened and subjected to spectral analysis as described above. Sleep architecture of the twins was compared to the group means of WS (N=20) and TD (N=20) subjects (data from ref. 15). Deviations from the confidence intervals were considered as significant alterations.
Results
GENETIC ANALYSES
Using the MLPA reaction the DNA sample of the twin brother showed reduced levels of amplification for all of the probes in the investigated region, thus he was a carrier of a typical deletion spanning at least 1.038 Mb between FKBP6 (7:7274216772772634) and CLIP2 (7:73703805-73820273). The deletion of genes outside this chromosomal region could not be tested with this assay. The results of the twin sister showed normal copy numbers in the investigated region.
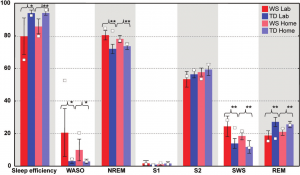
Figure 1. Individual sleep architectural values (%) of the twins visualized on box plots of group values (%). Individual values are open squares (o). Group values are based on the subjects involved in a previous study15. Significant group differences are indicated as follows: * p<.05, ** p<.01
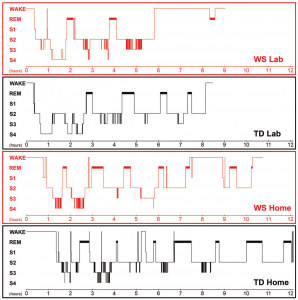 Figure 2. Hypnograms of the twins referring to laboratory and home sleep. The 1st and the 3rd graphs depict the sleep architecture of the laboratory and home sleep of the WS twin, respectively. The 2nd and the 4th graphs depict the laboratory and the home sleep of the TD twin, respectively
Figure 2. Hypnograms of the twins referring to laboratory and home sleep. The 1st and the 3rd graphs depict the sleep architecture of the laboratory and home sleep of the WS twin, respectively. The 2nd and the 4th graphs depict the laboratory and the home sleep of the TD twin, respectively
SLEEP ARCHITECTURE
When compared to his TD sister the WS twin had lower sleep efficiency, higher WASO, and higher SWS percent in the laboratory. In contrast the WS and the TD twins were characterized by similar NREM and REM sleep percents in their laboratory records (Figure 1.). The WS subject had only three sleep cycles during the night, while his TD sister had four. Differences in REM sleep latency might reflect a skipped first REM phase in the TD twin (Figure 2.). When using group averages as a reference (N=9 WS subjects) the sleep structure of the WS twin was close to the WS group means in terms of relative S1, S2, SWS and REM values. However, the WS twin had lower sleep efficiency as well as higher WASO and NREM sleep percent than the expected WS values. In contrast, the sleep architecture of the TD twin was close to the TD means (N=9 TD subjects) in terms of sleep efficiency, WASO and S1, but an increased NREM and SWS as well as decreased REM sleep was also observed (Figure 1.). The one year follow-up (ambulatory recording) confirmed the striking differences in sleep efficiency, WASO, and SWS between the twins (Figure 1.). It is interesting that both subjects had seven sleep cycles during their ambulatory home recordings (Figure 2.). Likewise in the laboratory records the twins had similar NREM and REM sleep values in the one year follow-up. When using WS and TD group means (20 WS and 20 TD subjects participating in ambulatory sleep recording studies) as references the WS twin had a more fragmented sleep (low sleep efficiency, high WASO) and lower S2 value than his counterparts. NREM sleep, S1, SWS and REM sleep were close to the expected WS values (Figure 1.). The sleep architectural measures of the TD twin were close to the expected values based on group means of ambulatory home-recorded sleep data of other TD subjects (Figure 1.).
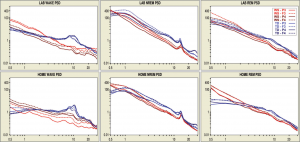 Figure 3. Double logarithmic plots of the EEG spectra in different behavioural states and recording nights. Red lines indicate plots of the WS twin, while blue lines indicate plots of the TD twin
Figure 3. Double logarithmic plots of the EEG spectra in different behavioural states and recording nights. Red lines indicate plots of the WS twin, while blue lines indicate plots of the TD twin
EEG SPECTRA
Wakefulness NREM sleep and REM sleep were characterized by striking stability in terms of their individual- and state-specific EEG spectra. This is evidenced by the visual inspection of Figure 3. As regarding the comparison of the overall EEG spectra of the twins, the 3 to 30 Hz power of the WS twin was lower than the 3–30 Hz power of the TD twin in both wakefulness and sleep. A clear-cut, state-independent difference in EEG power is evident for the frequencies roughly corresponding to the α band (8–12 Hz). State-specific spectral EEG differences between the twins are evident in the NREM sleep EEG σ (12–15 Hz) range. Spectral peak frequency (corresponding to NREM sleep EEG spindling) is higher in the WS than in the TD twin in both records. Moreover, a slowing of spindling in the frontal (anterior) derivations of the TD twin is evident in both records, while there is no sign for a similar phenomenon in the WS twin. As regarding higher frequencies during NREM sleep, the striking beta peak (~28 Hz) is present in the TD twin but not in the WS twin. Other state-specific differences are related to the lack of the αpeak in the presleep records of the WS twin. This contrasts the presence of the clear-cut α peaks of the TD twin in her presleep records. Differences in lower frequencies could be informative in NREM sleep, but these were not unequivocal. The WS twin had higher power in the range of the slow oscillation (<1 Hz) in some but not all derivations, while less or equal values in the 1–2 Hz range during NREM sleep were also evidenced.
SLEEP SPINDLE ANALYSIS
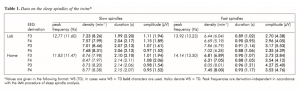
Results of the sleep spindle analyses are presented in Table 1. Siblings most consistently differed in sleep spindle frequency and amplitude. The frequency (Hz) of both slow and fast sleep spindles were higher in the WS than in the TD twin. In contrast spindles (slow and fast) of the WS twin were characterized by lower amplitude (µV) than spindles of the TD twin. Last, but not least shorter sleep spindles were characteristic for the WS twin in comparison with his TD sister. This latter difference was mostly expressed in the case of fast sleep spindles, and less so for slow spindles. Differences in slow and fast sleep spindle densities (min-1) were usually low and/or inconsistent across the nights.
Table 1. Data on the sleep spindles of the twins (Values are given in the following format: WS (TD). In cases were WS > TD boldcharacters are used. Italicsdenote WS < TD. Peak frequencies are derivation-independent in accordance with the IAM procedure of sleep spindle analysis.)
Discussion
Here we provide information on the differences and similarities between the sleep of a twin pair discordant for WS. Our aim was to replicate our previous statistical findings in an individualized analysis based on a case study in which psychosocial, socioeconomic and environmental factors are controlled. Moreover, we aimed to compare some sleep-wake variables which were not analyzed before in subjects with WS. These were the EEG spectra in wakefulness, detailed sleep spindle analysis as well as the separation of state-specific and state-independent EEG alterations. Reported WS-related macrostructural sleep alterations11, 13, 14 were reflected in the differences in sleep architecture between the twins. Lower sleep efficiency and higher WASO of the WS twin was evident in the laboratory as well as during the ambulatory home recording. Likewise the previously reported difference in SWS was supported by the higher relative SWS time of the WS twin during both laboratory and home sleep. In contrast the lower REM sleep values of WS subjects11, 13, 14 were not supported by our case study. Both laboratory and home records yielded equal percents of REM sleep time in the twins. In other words alteration in sleep continuity (low sleep efficiency, high WASO) as well as increased SWS seem to be core features of the disorder, while reduced REM sleep might be at least in part consequences of some non-specific environmental (sleep-related) or even non-WSrelated genetic factors. Obviously, the deliberate testing of this statement needs further empirical support and future studies. Our spectral EEG results were supportive for a strong reliability of this EEG measure as well as for a state-independently decreased EEG power in the α range. Although reports on decreased α power during NREM and REM sleep of WS patients were published before13, 15, no analyses focusing on wakefulness were carried out in these studies. According to our present case study the decreased EEG αpower in the WS twin is particularly evident during presleep wakefulness, when clear α peaks emerge in the EEG spectra of different derivations in the TD twin. No such peaks are seen in the WS twin. We can only speculate whether this striking decrease in EEG α waves is characteristic for a larger WS sample. Reports on decreased NREM and REM sleep α in WS13, 15 as well as the several strong state-independent, individual features in EEG power30 are suggestive for a general α EEG anomaly in WS. Given the strong evidences for the posterior (parietal and occipital) origin of α waves, as well as the relationship between α waves and visual information processing we could speculate that this EEG phenotype is a direct reflection of the parieto-occipital anomaly and visuospatial dysfunction in WS. A displacement of the σ spectral peak toward higher frequency in NREM sleep was evident in our present study. Together with the lack of a clear slow spindle (low σ) peak with anterior predominance this finding is coherent with the earlier reports15. The finding is consistent with the notion of the NREM sleep-dependent acceleration of the thalamocortical oscillatory dynamics in WS. No direct analyses of sleep spindles were performed in subjects with WS before. Here we provide the results of the IAM of sleep spindle analyses for the whole nights of NREM sleep in the twins. Analyses reveal faster sleep spindling with lower mean amplitude as well as shorter bursts of fast sleep spindles in the WS twin as compared to the TD twin. These findings cohere with the notion of an accelerated thalamocortical oscillatory dynamism in the NREM phase of sleep of subjects with WS15. Moreover, results of our current analyses of the amplitude of spindle events further strengthen the report of a decreased NREM sleep σ power in patients with WS13. It remains to be determined whether the reported shortening of the fast sleep spindle bursts of the WS twin is a general feature of WS or some accidental phenotype. It is worth noting, that the shortening of spindle bursts was found to be a potential biomarker of dementia among older subjects31, thus the cognitive deficits of WS subjects could be related to the shortening of sleep spindle bursts. Although our understanding about the association of the genetic alterations and the typical behavioural phenotypes in WS is fragmented, considerable effort has been made to delineate genotype-phenotype correlations in this genetic syndrome. Out of the 25–28 genes affected in WS, some are associated with the typical somatic alterations, i.e. connective tissue dysfunction, others remain unknown as of today in terms of biological function and disease pathology. It is conceivable that the neuropsychiatric alterations should be explicable by haploinsufficiency of genes shown to be strongly expressed in the developing or adult CNS by expression studies. Alternatively, these symptoms might also be caused by direct or indirect downstream effects, or possibly the interaction of single gene-related effects. WS genes expressed in the CNS include cytoplasmic linker 2 (CLIP2), syntaxin 1A (STX1A), frizzled 9 (Fz9D), and LIM domain kinase 1 (LIMK1)32. CLIP2 encodes a cytoplasmic linker protein responsible for interactions of intracellular organelles and the microtubule network. LIMK1 encodes a cytoplasmic protein kinase that plays a role as a regulator of cofilin-actin dynamics. These two genes are possible candidates for explaining structural and functional abnormalities in WS, as they both regulate cytoskeletal processes that influence neuronal structure, synaptic plasticity and axonal guidance. The protein coded by STX1A is connected to neurotransmitter release by synaptic docking. Although general transcription factor II-I repeat domain-containing 1 (GTF2IRD1) and general transcription factor II-Ii (GTF2I) were not covered by the assay used in this study, they should be mentioned as genes coding transcription factors of yet unknown functions. Atypical deletions can help to narrow the set of genes responsible for the neuropsychiatric alterations in WS. Several cases have been described with typical WS behavioural phenotypes but with smaller deletions sparing STX1A and all proximal genes32. These findings question the role of STX1A and FZD9 in the development of the behavioural and cognitive abnormalities. Dai et al33 described a patient with atypical deletions of the WBCR incorporating GTF2IRD1 but not GTF2I. Based on the detailed cognitive and behavioural analysis of this patient and her comparison to WS patients carrying typical deletions the authors suggested that GTF2IRD1 is associated with visuospatial deficits while GTF2I may be related to the typical social phenotype in WS. This hypothesis was backed by the finding that the mouse model lacking one copy of GTF2I demonstrates increased overly social behaviour34. Conversely, in autism that is connected with decreased social behaviour, genome-wide analysis revealed an increased prevalence of microduplications in the WBCR35. In spite of this marked contrasts in genetics (microdeletion vs. duplication) and social phenotype (hyper vs. hyposociability) of WS and autism, the sleep EEG profiles of the two syndromes share some commonalities, like increased WASO, decreased EEG αand σ power in the NREM phase4, 13 (see also the present findings). Imaging studies is WS patients showed interrelated structural and functional abnormalities including grey matter reductions bilaterally in the intraparietal sulcus and the orbitorfrontal (OFC) cortex, deficits in the dorsal visual stream and hippocampal activation, and the dissociation of OFC-amygdala interactions5. These results pointing to differentially altered neural connectivity, together with findings from WS animal models34, although on a solely speculative basis, might help to explain the perturbed sleep architecture and EEG oscillations identified in WS. To our knowledge this is the first twin study focusing on sleep of a subject with WS. Potential shortcomings of our approach are the discordance in sex of the twins. Gender differences in sleep EEG of adults between the ages of 20–60 years were reported in previous studies36, thus our findings are uncontrolled for this possibility. Among the sexually dimorphic sleep EEG features higher EEG power in females compared to males36 as well as menstrual cycle-related fluctuations in σ EEG frequency37 were reported before. These could contribute to our present results; however almost all of the differences reported here cohere with previous gender-matched statistical findings13–15. Moreover, we tested the repeatability of the results by recording sleep after one year in home settings. In sum, comparing polygraphic sleep data of the twins discordant for WS support the previously reported sleep architectural peculiarities of 7q11.23 micro deletion. Among these low sleep duration, reduced sleep efficiency, higher WASO and increased SWS of the WS twin was evident during both recording nights. In contrast previously reported differences in REM sleep percent were not supported in this twin study. Inter-twin EEG spectral differences strongly supported the thesis of a reduced α/σ power in WS. Together with the results of the sleep spindle analysis the above findings cohere with the notion of an accelerated thalamocortical oscillatory dynamics during NREM sleep in WS. Our report raised the possibility of a state-independent α EEG anomaly in WS, with potential relevance in the WSspecific visuospatial dysfunction. The relevance and replicability of lower amplitude and shorter sleep spindle bursts in WS waits for future research in the field.
ACKNOWLEDGEMENT The study was supported by the Hungarian National Science Found (OTKA-NF60806 to I.K. and OTKA-PD 83876 to JMR). The twins and all other members of their family kindly and patiently collaborated with us during the whole course of the study. Authors would like to express their appreciation and thanks for their patience and understanding during this process.
References:
1. Harvey MT, Kennedy CH. Polysomnographic phenotypes in developmental disabilities. Int J Dev Neurosci 2002; 20(3-5):443-8.
2. Philipsen A, Feige B, Hesslinger B, et al. Sleep in adults with attention-deficit/hyperactivity disorder: a controlled polysomnographic study including spectral analysis of the sleep EEG. Sleep 2005;28(7):877-84.
3. Doran SM, Harvey MT, Horner RH, Scotti J. Sleep and Developmental Disabilities: Assessment, Treatment, and Outcome Measures. Ment Retard 2006;44(1):13-27.
4. Lázár AS, Lázár ZI, Bíró A, et al. Reduced fronto-cortical brain connectivity during NREM sleep in Asperger syndrome: an EEG spectral and phase coherence study. Clin Neurophysiol 2010;121(11):1844-54.
5. Meyer-Lindenberg A, Mervis CB, Berman KF. Neural mechanisms in Williams syndrome: a unique window to genetic influences on cognition and behaviour. Nat Rev Neurosci 2006;7(5):380-93.
6. Järvinen-Pasley A, Bellugi U, Reilly J, et al. Defining the social phenotype in Williams syndrome: a model for linking gene, the brain, and behavior. Dev Psychopathol 2008; 20:1-35.
7. Bellugi U, Lichtenberger L, Jones W, Lai Z, St. George M. The neurocognitive profile of Williams Syndrome: a complex pattern of strengths and weaknesses. J Cogn Neurosci 2000;12(Suppl 1):7-29.
8. Karmiloff-Smith A, Grant J, Berthoud I, Davies M, Howlin P, Udwin O. Language and Williams Syndrome: How intact is ‘intact’? Child Dev 1997;68(2):246-62.
9. Leyfer OT, Woodruff-Borden J, Klein-Tasman BP, Fricke JS, Mervis CB. Prevalence of psychiatric disorders in 4-16year-olds with Williams syndrome. Am J Med Genet B, Neuropsychiatr Genet 2006;141B(6):615-22.
Morris CA, Mervis CB. Williams syndrome and related disorders. Annu Rev Genomics Hum Genet 2000;1:461-84.
11. Arens R, Wright B, Elliott J, et al. Periodic limb movement in sleep in children with Williams syndrome. J Pediatr 1998;133:670-4.
12. Goldman SE, Malow BA, Newman KD, Roof E, Dykens EM. Sleep patterns and daytime sleepiness in adolescents and young adults with Williams syndrome. J Intellect Disabil Res 2009;53:182-8.
13. Gombos F, Bódizs R, Kovács I. Atypical sleep architecture and altered EEG spectra in Williams Syndrome. J Intellect Disabil Res 2011;55(3):255-62.
14. Mason TB, Arens R, Sharman J, et al. Sleep in children with Williams Syndrome. Sleep Med 2011;12(9):892-7.
15. Bódizs R, Gombos F, Kovács I. Sleep EEG fingerprints reveal accelerated thalamocortical oscillatory dynamics in Williams syndrome. Res Dev Disabil 2012;33:153-64.
16. Hackman DA, Farah MJ. Socioeconomic status and the developing brain. Trends Cogn Sci 2009;13(2):65-73.
17. Hackman DA, Farah MJ, Meaney MJ. Socioeconomic status and the brain: mechanistic insights from human and animal research. Nat Rev Neurosci 2010;11:651-9.
18. Murphy MB, Greenberg F, Wilson G, Hughes M, DiLiberti J. Williams Syndrome in Twins. Am J Med Genet Suppl 1990;6:97-9.
19. Nakanishi T, Iwasaki Y, Momma K, Imai Y. Supravalvular aortic stenosis, pulmonary artery stenosis, and coronary artery stenosis in twins. Pediatr Cardiol 1996;17:125-8.
20. Castorina P, Selicorni A, Bedeschi F, Dalpra L, Larizza L. Genotype-Phenotype Correlation in Two Sets of Mono- zygotic Twins With Williams Syndrome. Am J Med Genet 1997;69:107-11.
21. Volterra V, Longobardi E, Pezzini G, Vicari S, Antenore C. Visuo-spatial and linguistic abilities in a twin with Wil- liams syndrome. J Intellect Disabil Res 1999;43(4):294305.
22. Pankau R, Siebert R, Kautza M, et al. Familial WilliamsBeuren Syndrome Showing Varying Clinical Expression. Am J Med Genet 2001;98:324-9.
23. De Gennaro L, Marzano C, Fratello F, et al.The electroencephalographic fingerprint of sleep is genetically determined: a twin study. Ann Neurol 2008;64(4):455-60.
24. Stuppia L, Antonucci I, Palka G, Gatta V. Use of the MLPA Assay in the Molecular Diagnosis of Gene Copy Number Alterations in Human Genetic Diseases. Int J Mol Sci 2012;13(3):3245-76.
25. Depienne C, Heron D, Betancur C, et al. Autism, language delay and mental retardation in a patient with 7q11 duplication. J Med Genet 2007;44(7):452-8.
26. Jasper HH. Report of the committee on methods of clinical examination in electroencephalography. Electroence- phalogr Clin Neurophysiol 1958;10:370-5.
27. Kemp B, Värri A, Rosa AC, Nielsen KD, Gade J. A simple format for exchange of digitized polygraphic recordings. Electroencephalogr Clin Neurophysiol 1992;82:391-3.
28. Rechtschaffen A, Kales A. Manual of Standardized Termi- nology, Techniques and Scoring System for Sleep Stages of Human Subjects. Los Angeles: UCLA Brain Inform a- tion Service/Brain Research Institute, 1968.
29. Bódizs R, Körmendi J, Rigó P, Lázár AS. The individual adjustment method of sleep spindle analysis: Methodo- logical improvements and roots in the fingerprint paradigm. J Neurosci Methods 2009;178:205-13.
30. Tinguely G, Finelli LA, Landolt HP, Borbély AA, Acher mann P. Functional EEG topography in sleep and waking: state-dependent and state-independent features. Neuro- image 2006;32(1):283-92.
31. Ktonas PY, Golemati S, Xanthopoulos P, et al. Potential dementia biomarkers based on the time-varying micro- structure of sleep EEG spindles. Conf Proc IEEE Eng Med Biol Soc 2007;2007:2464-7.
32. Merla G, Brunetti-Pierri N, Micale L, Fusco C. Copy number variants at Williams-Beuren syndrome 7q11.23 region. Hum Genet 2010;128(1):3-26.
33. Dai L, Bellugi U, Chen XN, et al. Is it Williams syndrome? GTF2IRD1 implicated in visual-spatial construction and GTF2I in sociability revealed by high resolution arrays. Am J Med Genet A 2009;149A(3):302-14.
34. Osborne LR. Animal models of Williams syndrome. Am J Med Genet C Semin Med Genet 2010;154C(2):209-19.
35. Sanders SJ, Ercan-Sencicek AG, Hus V, et al. Multiple recurrent de novo CNVs, including duplications of the 7q11.23 Williams syndrome region, are strongly associated with autism. Neuron 2011;70(5):863-85.
36. Carrier J, Land S, Buysse DJ, Kupfer DJ, Monk TH. The effects of age and gender on sleep EEG power spectral density in the middle years of life (ages 20-60 years old). Psychophysiology 2001;38(2):232-42.
37. Driver HS, Dijk DJ, Werth E, Biedermann K, Borbély AA. Sleep and the sleep electroencephalogram across the menstrual cycle in young healthy women. J Clin Endocrinol Metab 1996;81:728-35.