Sleep Breath (2016) 20:1045–1051
DOI: 10.1007/s11325-016-1347-7
Johanna Takács1 & Róbert Bódizs1,2 & Péter Przemyslaw Ujma1 & Klára Horváth3 & Péter Rajna4 & László Harmat5
1 Institute of Behavioural Sciences, Semmelweis University, H-1089 Budapest, Nagyvárad tér 4, Hungary
2 Department of General Psychology, Pázmány Péter Catholic University, Budapest, Hungary
3 2nd Department of Pediatrics, Semmelweis University, Budapest, Hungary
4 Department of Psychiatry and Psychotherapy, Semmelweis University, Budapest, Hungary
5 Department of Neuroscience, Karolinska Institutet, Stockholm, Sweden
Abstract
Purpose: The Pittsburgh Sleep Quality Index is used to evaluate subjective sleep quality, and it is commonly used in clinical research. Subjective sleep quality is also an important clinical measure in patients with psychiatric disorders. The aim of the present study was to evaluate the reliability and validity of the Hungarian version of the Pittsburgh Sleep Quality Index (PSQI-HUN) in both clinical and non-clinical samples.
Methods: The original version of PSQI was translated into Hungarian according to standard guidelines. The PSQI-HUN and the Athens Insomnia Scale (AIS) were subsequently administered to 53 psychiatric patients (schizophrenia, recurrent depressive disorder, mixed anxiety, and depressive disorder) and 178 healthy controls.
Results: Internal consistency as measured by Cronbach’s alpha in the whole sample was 0.79. Pearson’s product-moment correlations between component scores and the global scores were high (0.59–0.88) in the PSQI-HUN indicating the homogeneity of the scale. PSQI-HUN global and component scores differed significantly between psychiatric patients and control subjects. In the psychiatric patient subsample, schizophrenics had lower global scores compared to the other two patient groups. The analysis of convergent validity showed significant correlations between the AIS and the global as well as the component scores of the PSQI-HUN (except the component of sleep latency).
Conclusions: The present study concludes that the PSQI-HUN is a reliable, valid, and standardized measure for assessment of the subjective sleep quality in clinical and research settings.
Keywords: Pittsburgh Sleep Quality Index . Reliability .Validity . Sleep disorders . Psychiatric disorders
Introduction
Since its introduction in 1989, the Pittsburgh Sleep Quality Index (PSQI) [1] has gained widespread acceptance as a useful tool to measure sleep quality. Subjective assessment of sleep quality and disturbance is a topic of significant importance for a large number of researchers and clinicians because diminished sleep quality and the presence of sleep disturbance can profoundly impact the quality of life and may be associated with somatic and psychological complaints [1,2]. The PSQI has been widely used in various lines of clinical research, since poor subjective sleep quality may be a possible indicator of major depressive disorder and may also predict suicidal tendencies in patients with major depression [3]. Subjective sleep quality is also an outcome measure in elderly people with sleep complaints or depression [4]. Sleep disturbance and poor sleep quality may be the result of medication side effects, physical discomfort, or other aspects of physical illness, as well as psychiatric disorders such as depression, anxiety disorders, and schizophrenia [2]. The PSQI was originally designed for clinical purposes, in order to give a valid assessment of both sleep quality and disturbances. PSQI is used in the assessment of subjective sleep dysfunction over a 1-month period but may also be used as a tool to measure weekly changes in sleep quality [5,6].
The PSQI has been translated into several languages, including French, Japanese [3], German, Spanish, Chinese, and Hebrew [7]. Doi and colleagues [3] found an overall reliability coefficient (Cronbach’s alpha) of 0.77 in a study of psychiatric patients and control subjects. Backhaus at al. [8] measured the test-retest reliability and validity of PSQI in primary insomnia and reported a Cronbach’s alpha score of 0.85 and high test-retest reliability (0.87). For the Hebrew version of PSQI [7], Cronbach’s alpha scores for clinical and non-clinical samples were 0.70 and 0.52, respectively, and 0.72 for the two groups combined. These studies found significant group differences (patients vs. controls) in the average global and component PSQI scores. Furthermore, their results demonstrated the adequate reliability and validity of the PSQI in both clinical and non-clinical populations.
So far, no previous studies have investigated the reliability and validity of PSQI in the Central-Eastern European region, despite the fact that the scale has already been translated into more than to 56 languages. We developed a Hungarian version of the Pittsburgh Sleep Quality Index (PSQI-HUN), and the goals of the present study were to assess the internal reliability and concurrent validity of the PSQI-HUN in both clinical and non-clinical samples using the traditional single factor of the PSQI. We also measured the discriminant validity of the components of the PSQI-HUN as well as its convergent validity with regard to the Hungarian version of Beck Depression Inventory (BDI) [9], Athens Insomnia Scale (AIS) [10,11], and Spielberger Trait Anxiety Scale (STAI-T) [12] subscores. We hypothesized that PSQI-HUN global score as well as component scores would be higher in patients with psychiatric disorders than in healthy controls without sleep complaints and that PSQI-HUN global score would correlate with the AIS global score.
Methods
Participants
Two-hundred thirty-one participants were included to the whole study (mean age ± SD 43.5 ± 9.1 years, 128 females). The clinical sample included 53 inpatients and outpatients referred to the Nyírő Gyula Hospital, Department of Psychiatry, Budapest, Hungary (mean age ± SD 46.07 ± 9.92 years, 33 females). Clinical evaluations of the subjects were carried out by psychiatrists at Nyírő Gyula Hospital, Department of Psychiatry, Budapest, Hungary, and included a complete medical history and a clinical interview. Considering these clinical findings, final diagnoses were made based on Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria [13]. Patients were assigned to one of the three groups: (1) recurrent depressive disorder (n = 23; mean age ± SD 47.35 ± 6.99 years, 15 female), (2) schizophrenia (n = 15; mean age ± SD 45.87 ± 9.92 years, 6 female), and (3) mixed anxiety and depressive disorder (n = 15; mean age ± SD 44.33 ± 13.56 years, 12 female). The Hungarian version of PSQI (PSQI-HUN) was administered to the clinical sample once.
One-hundred seventy-eight participants (mean age ± SD 42.77 ± 8.77 years, 95 female) were recruited for the control group through advertisement, and they filled out the questionnaires online. The control group completed the PSQI-HUN two times over the course of 4 weeks to assessment performance consistency. In addition, these participants also filled out AIS, BDI, and STAI-T in order to determine convergent validity. Participants with a PSQI-HUN score over 5 or/and who a BDI score over 12—that is, with moderate and severe depressive symptoms [14]—or being under medical or psychiatric treatments were excluded from the control group.
There were no significant differences in gender (χ 2 (3, 231) = 6.280, p = 0.099) and age (F (3,227) = 2.149, p = 0.095) between the control and psychiatric patient groups.
Assessment
The PSQI consists of 19 items that produce an estimate of global sleep quality based on the following seven components: perceived sleep quality, sleep latency, sleep duration, sleep efficiency, sleep disturbances, hypnotic medication use, and daytime dysfunctions. Each component score ranges from 0 to 3 that yield a global score that ranges between 0 and 21. A global PSQI score greater than 5 indicates that a subject is experiencing serious complaints in at least two sleep quality parameters. This threshold gives a diagnostic sensitivity of 89.6 % when distinguishing between good and poor sleepers. Age and sex do not correlate strongly with PSQI component scores [1].
The PSQI-HUN was developed by first translating the original PSQI from English into Hungarian and then, using an independent translator, back into English. After this, the original and the back-translated PSQI versions were compared and a preliminary Hungarian version was created.
The participants’ perception of anxiety was measured with the STAI [12]. The instrument consists of two scales which measure the distinct concepts of state and trait anxiety. Trait anxiety reflects a stable predisposition to anxiety as determined by the personality profile. State anxiety is a temporary emotional response to a stressful situation. Both scales consist of 20 items with responses given on a four-point Likert scale. The total score is the weighted sum of all 20 responses and ranges from 20 to 80. The cut points were low anxiety 20–30, moderate anxiety 40–59, and high anxiety 60–80. Scores were found to be considerably higher under stress than under normal conditions [15]. Test-retest reliability correlations reported in the literature for the trait anxiety scale range from 0.73 to 0.86, while correlations for the state anxiety scale vary between 0.16 and 0.54 [12]. We used the Hungarian version of the trait anxiety scale (STAI-T) in our study [16].
Depressive symptoms were assessed by the BDI [9]. We used a Hungarian translation of the shortened version of BDI [17]. The shortened version of BDI consists of 13 items and has been shown to have an internal consistency of α = 0.85 in a nationwide representative Hungarian sample [14]. The BDI and its shortened versions are reliable methods of measuring the severity of the depressive syndrome. Scores correlate reliably with the severity of depressive symptomatology estimated by psychiatrists [18]. Items of the short form of BDI are related to the following characteristics of depression: sadness, pessimism, sense of failure, dissatisfaction, guilt, self-dislike, self-harm, social withdrawal, indecisiveness, self-image, work difficulty, fatigue, and appetite [17].
The AIS is a self-assessment psychometric instrument designed for quantifying sleep difficulty based on DSM-IV and ICD-10 criteria [10]. It consists of the following eight items: the first five pertain to sleep induction, awakenings during the night, final awakening, total sleep duration, and sleep quality, while the last three assess well-being, functioning capacity, and sleepiness during the day. Both the entire eight-item scale (AIS-8) and a brief five-item version (AIS-5), which contains only the first five items, are available. The validation of the AIS was based on its administration to 299 subjects, 105 primary insomniac patients, 144 psychiatric patients, and 50 non-patient controls [10,11]. Regarding internal consistency for both versions of the scale, the Cronbach’s alpha was around 0.90 and the mean item-total correlation coefficients were about 0.70. Moreover, in the factor analysis, a single-factor component was revealed. The test-retest reliability correlation coefficient was found to be almost 0.90 at a 1-week interval. As far as external validity is concerned, the correlations of the AIS-8 and AIS-5 with the Sleep Problems Scale were 0.90 and 0.85, respectively [10]. We used the Hungarian version of the AIS-8, which shows high internal consistency (Cronbach’s alpha = 0.86) and good overall test reproducibility (r = 0.72) [19].
Statistical analysis
We evaluated the psychometric properties of the PSQI-HUN in psychiatric and control subjects using the following measures of reliability and validity: internal consistency, homogeneity of the scale, performance consistency (test-retest variability), criterion validity, and convergent validity.
Internal consistency (Cronbach’s alpha) was computed for the psychiatric patients and for the control group both separately and in combination. Pearson’s product-moment correlation coefficients indicating the relationship between the global and component scores, as well as corrected component-item correlation coefficients, were calculated in the clinical and the control samples in order to evaluate the homogeneity of the scale.
Test-retest reliability analysis was performed for the scores from the control subjects (n = 178). PSQI-HUN global scores, component scores, and items at time 1 (T1) and time 2 (T2) were compared by paired t tests and the extent of their parallel change tested by Pearson’s product-moment correlations. T2-T1 was roughly 1 month (between February 1 and March 10, 2015).
Criterion validity was tested by comparing the scores of the clinical and the control groups, by using analysis of covariance (ANCOVA) on PSQI-HUN components and global scores after adjusting for gender and age and correcting for multiple comparisons among the groups by the Bonferroni correction method.
We calculated Pearson’s product-moment correlation coefficients between PSQI-HUN scores (global and components scores) and AIS, BDI, and STAI-T scores in the control group in order to evaluate convergent validity. All analyses were performed using SPSS version 20 (IBM SPSS Statistics, IBM Corporation, Chicago, IL).
Results
Statistical analysis of reliability
Internal consistency. Cronbach’s alpha coefficient in the full sample (that is, including all subjects in all groups) was α = 0.79. A subgroup analysis resulted in a Cronbach’s alpha of 0.52 for the control group and 0.78 for the clinical group. Clinical subgroups were characterized by Cronbach’s alpha values of 0.79 for patients with recurrent depressive disorder, 0.75 for patients with schizophrenia, and 0.78 for patients with mixed anxiety and depressive disorder.
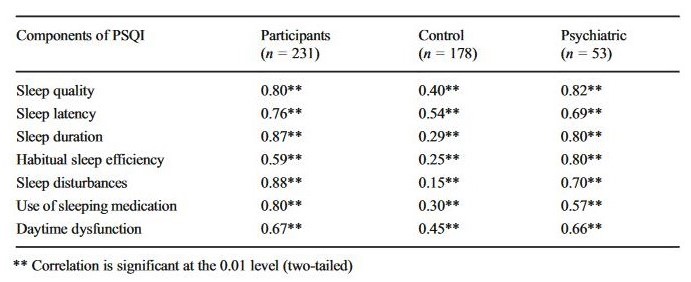
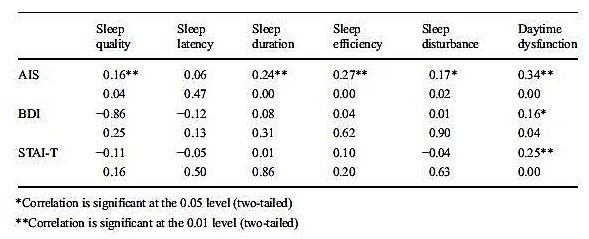
Homogeneity of the scale. Pearson’s product-moment correlations between component scores and the PSQI-HUN global score are shown in Table 1. Significant correlations were observed for all components and ranged between 0.59 and 0.88 (p < 0.001). The strongest correlations were found for sleep disturbances (r = 0.88, p < 0.001), sleep duration (r = 0.87, p < 0.001), and sleep quality (r = 0.80, p < 0.001).
Table 1
Pearson’s product-moment correlations between component scores and PSQI-HUN global score in the total sample and in control/psychiatric subjects separately
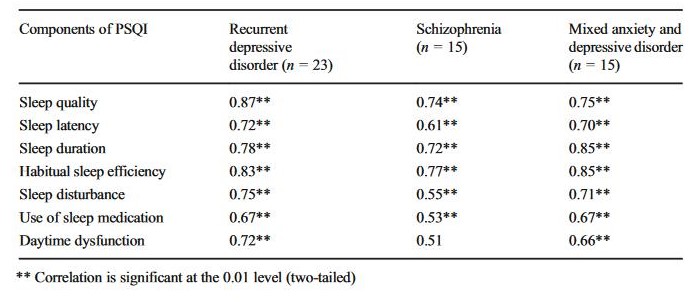
The homogeneity of the scale was tested for the control groups and the clinical groups separately. Correlation coefficients are shown in Tables 1 and 2. The strongest correlations in the control group were with sleep latency (r = 0.54, p < 0.001) and daytime dysfunction (r = 0.45, p < 0.001). In the clinical group, sleep quality (r = 0.82, p < 0.001), sleep duration (r = 0.80, p < 0.001), and habitual sleep efficiency (r = 0.80, p < 0.001) showed the strongest correlation with the PSQI-HUN global score. In the patient groups with the diagnosis of recurrent depressive disorder and schizophrenia, the strongest correlations were with sleep quality (r = 0.87, p < 0.001 and r = 0.74, p = 0.002, respectively) and habitual sleep efficiency (r = 0.83, p < 0.001 and r = 0.77, p = 0.001, respectively); the correlation between daytime dysfunction and global PSQI-HUN score was not significant in the schizophrenia group (r = 0.51, p = 0.052). Last, but not least, the high correlations of PSQI-HUN global scores with sleep duration (r = 0.85, p < 0.001) and habitual sleep efficiency (r = 0.85, p < 0.001) were characteristic features of mixed anxiety and depressive disorder patients.
Table 2
Pearson’s product-moment correlations between components and PSQI-HUN global score in psychiatric groups
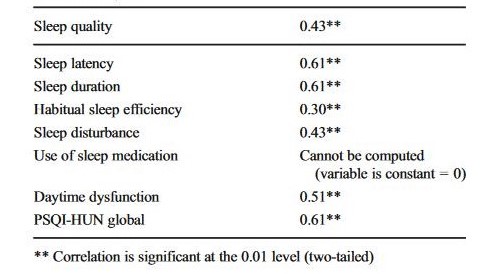
Performance consistency (4-week test-retest reliability). Results are shown in Table 3. The paired t test between T1 and T2 for the global PSQI-HUN score showed no significant differences (t (177) = 1.043; p = 0.298). Pearson’s product-moment correlations were stable in both global and component scores. The T1 vs. T2 correlation coefficient for PSQI-HUN global scores was 0.61 (p < 0.001). Component score correlation coefficients ranged between 0.61 (sleep latency and sleep duration) and 0.30 (habitual sleep efficiency; p < 0.01).
Table 3
PSQI-HUN component and global score correlations at T1-T2 in control subjects
Statistical tests of questionnaire validity
Criterion validity. Pairwise comparisons of PSQI-HUN scores of the control and the clinical groups (Bonferroni corrected) are shown in Table 4. PSQI-HUN global scores were significantly higher in the clinical group. Furthermore, the schizophrenia subgroup had lower global scores compared with the other two clinical groups. PSQI-HUN component scores were also significantly different between the clinical and the control groups; they were lower in the control group than in the clinical group. Among the latter, schizophrenia patients were characterized by the lowest values in most of the PSQI-HUN component scores.
Table 4
PSQI-HUN component scores and PSQI-HUN global score in control and psychiatric groups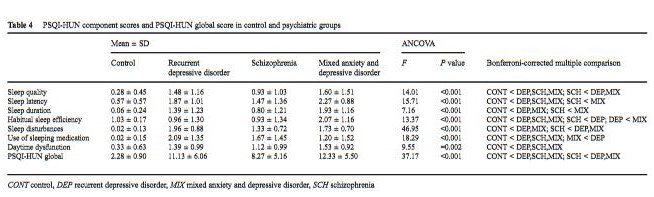
CONT control, DEP recurrent depressive disorder, MIX mixed anxiety and depressive disorder, SCH schizophrenia
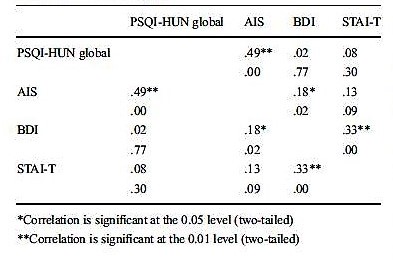
Convergent validity. Significant correlations between PSQI-HUN global scores and AIS scores (r = 0.49, p < 0.001; Table 5) were revealed. Moreover, sleep quality (r = 0.16, p = 0.036), sleep duration (r = 0.24, p = 0.001), sleep efficiency (r = 0.27, p < 0.001), sleep disturbances (r = 0.17, p = 0.022), and daytime dysfunction (r = 0.34, p < 0.001) were also correlated with AIS scores (Table 6). There was no significant correlation between sleep latency and AIS score (r = 0.05, p = 0.468). Only one component of PSQI-HUN was correlated significantly with BDI and STAI-T, namely, daytime dysfunction (BDI vs. daytime dysfunction 0.16, p = 0.037; STAI-T vs. daytime dysfunction 0.25, p = 0.001).
Table 5
Correlations between PSQI-HUN global score and AIS, BDI, and STAI-T in control subjects
Table 6
Correlations between PSQI-HUN component scores and AIS, BDI, and STAI-T in control subjects
Discussion
We investigated the reliability and validity of the PSQI-HUN in psychiatric patients and control subjects. Our findings strongly support our previous hypothesis that PSQI-HUN global scores and component scores are higher in patients with psychiatric disorders than in healthy controls without sleep complaints and that PSQI-HUN global scores correlate with AIS global scores. These results show that the PSQI-HUN measures a similar construct as the AIS. The scale separates subjective sleep quality and sleep complaints from the perception of anxiety or depressive symptoms (as evidenced by low, non-significant correlations with STAI and BDI).
The internal homogeneity of the PSQI-HUN was shown to be higher than in previous studies [1,3]. Cronbach’s alpha for PSQI-HUN was 0.79 for all subjects, 0.79 for recurrent depressive disorder, 0.75 for schizophrenia, 0.78 for mixed anxiety and depressive disorder, and 0.52 for control subjects. Our results are similar to the results of other studies [1, 3, 20, 21, 22, 23]. Similar to Doi et al. [3], we found a low internal consistency for PSQI-HUN global scores in control subjects (α = 0.52). In another study, Doi and colleagues [24] suggested that these results may be due to the fact that in control subjects, the component of sleeping medication use shows zero variance, which is the same way in our study.
Our results suggest that PSQI-HUN subscales are homogeneous. Pearson’s product-moment correlations between component scores and the global score were significant for all components. The strongest component vs. global score correlations were found for sleep latency and daytime dysfunction in control subjects. A somewhat lower correlation was found for the sleep disturbance component. In psychiatric subjects, all components showed particularly strong correlations. However, the statistical power of the three different subgroups of the clinical sample was lower than in the control group. Sleep quality, sleep duration, and habitual sleep efficiency showed the strongest correlations with global PSQI-HUN scores in patient groups with recurrent depressive disorder and schizophrenia. In turn, the strongest correlations between component scores and global score were in case of sleep duration and habitual sleep efficiency in patients with mixed anxiety and depressive disorder. The component which had the highest correlation with the global score across patient groups was sleep quality, while the least correlated components were the use of sleep medication and daytime dysfunction. These results are in line with the findings of Doi et al. 2000 [3] and Moghaddam et al. 2012 [22]. In addition, we also evaluated the performance consistency of the PSQI-HUN in the control group. The test-retest reliability measures showed strong stability in global scores as well as in six of the seven component scores across a 4-week test-retest session. These results are in line with Sohn et al. 2012 [23], who reported a test-retest correlation coefficient of 0.65 for the total score.
We also investigated the criterion validity of the PSQI-HUN in both psychiatric patients and control subjects. As hypothesized, the PSQI-HUN was discriminated between psychiatric patients and control subjects. More specifically, we found that both the global score and the component scores of the PSQI-HUN were significantly lower in the control group compared to the clinical sample. Doi and colleagues [24] found a significant difference only in case of one component of the PSQI (subjective sleep quality) when comparing clinical and non-clinical groups. In addition, PSQI-HUN component scores were significantly lower in the schizophrenia group compared to the other psychiatric patient groups. The total score of the schizophrenic group was similar to the results reported by Royuela et al. 2002 [25]. They found that schizophrenia patients have lower sleep quality compared to healthy controls and negative symptoms could in part reflect the deterioration of the patients’ sleep quality [25]. However, these symptoms might be more severe in recurrent depressive and mixed anxiety and depressive disorder than in schizophrenia. In subjects with recurrent depressive disorder, sleep duration, the usage of sleep medication, and habitual sleep efficiency were significantly lower than in subjects with mixed anxiety and depressive disorder. These findings might indicate that the PSQI-HUN is able to differentiate between these psychiatric groups.
In addition, we investigated the convergent validity of the PSQI-HUN in control subjects. In Hungary, the screening of sleep disorders is usually based on the AIS and the Berlin questionnaire. The former is an appropriate tool for the assessment of the severity of insomnia, but the PSQI-HUN provides a more differentiated view of the severity of subjective sleep impairment in various psychiatric patient groups; hence, the Hungarian version of the PSQI might be useful in screening, clinical diagnostics, and follow-up.
There were some notable limitations of the present study. First, we have no detailed information on pharmacotherapy and other therapeutic interventions in clinical groups. Therefore, we cannot exclude that the differences in sleep quality between non-clinical and clinical subjects were affected specifically by these factors. It must be noted, however, that all patients were treated by psychiatrists belonging to the same staff. Psychotropic drugs were applied in accordance with the valid protocols. It meant SSRI or dual antidepressants in recurrent depressive disorder, second-generation antipsychotic drugs in schizophrenia and SSRI, and/or anxiolytic drugs (mainly BZD-s) in mixed anxiety and depressive disorder. All medications were applied within the accepted dose range. We can therefore expect that our patients’ performance in the PSQI-HUN can be considered typical for people suffering from (and treated for) similar psychiatric illnesses.
Second, the sample size of the clinical group was significantly smaller compared to the control group. However, our results are still consistent with Moghaddam et al. study (2012), where a total of 125 psychiatric patients’ sleep quality was investigated using PSQI. In spite of this fact, our results may not be generalizable to a wider psychiatric population, but we can suggest that PSQI-HUN is a useful measure for the assessment of subjective sleep quality in clinical management. Third, there are some potentially confounding factors between psychiatric and control subjects, such as the consumption of caffeinated beverages (tea, coffee, carbonated soft drinks) and cigars/cigarettes. There was no significant difference between the control group and psychiatric patients in terms of caffeinated beverage consumption, and this measure also failed to show a significant correlation with PSQI global and component scores either in the clinical or the control sample. The consumption of cigarettes differed significantly between the control group and psychiatric patients—subjects in control group consumed fewer cigarettes per day than in clinical groups. However, there was also no significant correlation between cigarette consumption and PSQI global or component scores either in the clinical or the control sample. In sum, even considering the limitations of this study, our findings indicate that the PSQI-HUN is a reliable and valid standardized tool for evaluating subjective sleep quality in clinical and research settings.
Notes
Acknowledgments
Authors gratefully acknowledge Örjan de Manzano and Miriam Mosing for their comments and proof reading on the manuscript.
Compliance with ethical standards
Conflict of interest
There are no potential or actual conflicts of interest.
Ethical approval
All procedures were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the Human Research Ethics Committee of the study university (Semmelweis University, Budapest, Hungary, 9094–4/2013/EKZ (125/2013)). Participants received both oral and written information about the study and they signed an informed consent form.
Informed consent
Informed consent was obtained from all individual participants included in the study.
References
1.Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ (1989) The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 28:193–213
2.Carpenter JS, Andrykowski MA (1998) Psychometric evaluation of the Pittsburgh Sleep Quality Index. J Psychosom Res 45:5–13
3.Doi Y, Minowa M, Uchiyama M, Okawa M, Kim K, Shibui K, Kamei Y (2000) Psychometric assessment of subjective sleep quality using the Japanese version of the Pittsburgh Sleep Quality Index (PSQI-J) in psychiatric disordered and control subjects. Psychiatry Res 97:165–172
4.Singh NA, Clements KM, Fiatarone MA (1997) A randomized controlled trial of the effect of exercise on sleep. Sleep 20:95–101
5.Harmat L, Takacs J, Bodizs R (2008) Music improves sleep quality in students. J Adv Nurs 62:327–335. doi: 10.1111/j.1365-2648.2008.04602
6.Lai HL (2005) Self-reported napping and nocturnal sleep in Taiwanese elderly insomniacs. Public Health Nurs 22:240–247
7.Shochat T, Tzischinsky O, Oksenberg A, Peled R (2007) Validation of the Pittsburgh Sleep Quality Index Hebrew translation (PSQI-H) in a sleep clinic sample. Isr Med Assoc J 9:853–856
8.Backhaus J, Junghanns K, Broocks A, Riemann D, Hohagen F (2002) Test-retest reliability and validity of the Pittsburgh Sleep Quality Index in primary insomnia. J Psychosom Res 53:737–740
9.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J (1961) An inventory for measuring depression. Arch Gen Psychiatry 4:561–571
10.Soldatos CR, Dikeos DG, Paparrigopoulos TJ (2000) Athens insomnia scale: validation of an instrument based on ICD-10 criteria. J Psychosom Res 48:555–560
11.Soldatos CR, Dikeos DG, Paparrigopoulos TJ (2003) The diagnostic validity of the Athens insomnia scale. J Psychosom Res 55:263–267
12.Spielberger CD (1972) Anxiety as an emotional state. In: Spielberger CD (ed) Anxiety: current trends in theory and research. Academic Press, New York, pp. 29–49
13.American Psychiatric Association (1994) Diagnostic and statistical manual of mental disorders: DSM-IV, 4th edn. Washington, DC
14.Rózsa S, Szádóczky E, Füredi J (2001) A Beck Depresszió Kérdőív rövidített változatának jellemzői hazai mintán. Psychiatr Hung 16:379–397
15.Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA (1983) Manual for state-trait anxiety inventory. Consulting Psychologists Press, Palo Alto, CA
16.Sipos K, Sipos M (1983) The development and validation of the Hungarian form of the state-trait anxiety inventory. Ser Clin C 2:27–39
17.Beck AT, Beck RW (1972) Shortened version of BDI. Post Grad Med 52:81–85
18.Al-Yasiri AR, AbdKarkosh YS (2013) The validity of Beck depression inventory—short version in depressed patients diagnosed according to ICD10. IPMJ 12:603–613
19.Novak M, Mucsi I, Shapiro CM, Rethelyi J, Kopp MS (2004) Increased utilization of health services by insomniacs—an epidemiological perspective. J Psychosom Res 56:527–536
20.Nunnally J, Bernstein I (1994) Psychometric theory, 3rd edn. McGraw-Hill, New York
21.Bertolazi AN, Fagondes SC, Hoff LS, Dartora EG, Miozzo IC, de Barba ME, Barreto SS (2011) Validation of the Brazilian Portuguese version of the Pittsburgh Sleep Quality Index. Sleep Med 12:70–75. doi: 10.1016/j.sleep.2010.04.020
22.Moghaddam JF, Nakhaee N, Sheibani V, Garrusi B, Amirkafi A (2012) Reliability and validity of the Persian version of the Pittsburgh Sleep Quality Index (PSQI-P). Sleep Breath 16:79–82. doi: 10.1007/s11325-010-0478-5
23.Sohn SI, Kim DH, Lee MY, Cho YW (2012) The reliability and validity of the Korean version of the Pittsburgh Sleep Quality Index. Sleep Breath 16:803–812. doi: 10.1007/s11325-011-0579-9
24.Doi Y, Minowa M, Okawa M, Uchiyama M (2000) Prevalence of sleep disturbance and hypnotic medication use in relation to sociodemographic factors in the general Japanese adult population. J Epidemiol 10:79–86
25.Royuela A, Macias J, Gil-Verina J, et al. (2002) Sleep in schizophrenia: a preliminary study using the Pittsburgh Sleep Quality Index. Neurobiol Sleep Wakefulness Cycle 2:37–39
Copyright information
© Springer-Verlag Berlin Heidelberg 2016