Physiology & Behavior 147 (2015) 233–237
Link
DOI: 10.1016/j.physbeh.2015.05.001
Tamás Nagya,⁎, Gyöngyvér Salaveczb, Péter Simorc,d, György Pureblb, Róbert Bódizsb, Samantha Dockraye, Andrew Steptoef
a Doctoral School of Psychology, Eötvös Loránd University, Izabella u. 46, H-1064 Budapest, Hungary
b Institute of Behavioral Sciences, Semmelweis University, Nagyvárad tér 4, H-1089 Budapest, Hungary
c Department of Cognitive Science, Budapest University of Technology and Economics, Egry József utca 1, H-1111 Budapest, Hungary
d Nyírő Gyula Hospital, National Institute of Psychiatry and Addictions, Lehel utca 59 H-1135 Budapest, Hungary
e School of Applied Psychology, University College Cork, Coláiste Na Hollscoile, Bóthar an Choláiste, Corcaigh, Ireland
f Department of Epidemiology and Public Health, University College London, 1-19 Torrington Place, WC1E 6BT London, United Kingdom
⁎ Corresponding author. E-mail address: nagy.tamas@ppk.elte.hu (T. Nagy).
Keywords: Cortisol awakening response; Hypothalamus pituitary adrenal axis; Nightmare; Sleep
Highlights
- Daily cortisol patterns were assessed on a working and leisure day in 188 women.
- The cross-sectional sample included 13 participants with frequent nightmares.
- Cortisol awakening response was smaller in the nightmare subgroup on the working day.
- Findings implicate that nightmares can be associated with HPA functioning.
Abstract
Nightmares are relatively common sleep complaints that seem to be associated with affective distress. To date, few attempts have been made to link nightmares to the biological markers of the stress response, and the HPA response in particular. The present study examined the relationship between frequent nightmares and the cortisol awakening response (CAR) in a cross-sectional study of working women (N = 188). Analysis revealed that those who reported frequent nightmares (N = 13) showed a blunted CAR on a working day, compared to those who did not report nightmares. This result was independent of psychiatric symptoms, demographic variables, and lifestyle. Our preliminary findings suggest that decreased HPA reactivity might be a trait-like feature of women with frequent nightmares.
- Introduction
Approximately 5% of the adult population suffers regularly from nightmares — vivid and terrifying dreams that lead to abrupt awakenings [1]. Still, relatively little is known about the pathogenesis of nightmares and its relation to sleep and mental disorders [2]. Within the frames of the “continuity hypothesis”, Schredl suggested an association between stressful life experiences and nightmares [3]. Whereas, questionnaire-based studies emphasize the relevance of state-like effects (such as increased emotional pressure) leading to frequent nightmares [3,4], theoretical models [2] as well as longitudinal [5] and twin studies e.g. [6] point to the influence of trait-like vulnerability factors for the development of frequent nightmares. In their multilevel, integrative model Levin and Nielsen [2] proposed that state-like affect load as well as trait-like affect distress might contribute to the frequent occurrence of terrifying dream experiences. Both factors seem to be related to increased emotional reactivity underlain by impaired fronto-limbic circuitry [2,7], and presumably abnormal stress responses. Unfortunately, previous studies have mostly relied on reports of subjective stress, and few nightmare studies used biological stress markers. Nonetheless, indirect evidence suggests an association between the HPA axis activity and nightmares. For instance, some of the brain regions implicated in nightmare formation [8] – the hippocampus, the amygdala and the medial prefrontal cortex in particular – abundantly express glucocorticoid receptors, influence HPA axis activity and regulate stress responses [9]. Additionally, the cortisol awakening response (CAR) – which refers to the sharp increase in cortisol following awakening and indicates the reactivity of the HPA system – has been negatively correlated with impaired sleep quality [10]. On the other hand, frequent nightmares have also been associated with impaired sleep quality [1], reduced sleep efficiency, increased nocturnal awakenings, relatively decreased slow wave sleep (SWS) and increased REM pressure [see [1]]. Moreover, several studies have shown that PTSD – a severe psychiatric condition in which affected individuals often experience vivid nightmares – is related to a blunted CAR [2,12–14]. The above findings led us to the assumption that the altered functioning of the HPA system might contribute to the pathogenesis of frequent nightmares. In particular, in light of the previously reported associations between sleep disturbances, PTSD symptoms and reduced CAR we expected that frequent nightmares would be associated with a blunted CAR. This hypothesis was examined within a non-clinical sample of women who provided seven cortisol samples through a working and a leisure day.
- Methods
This article reports on analyses performed on the Hungarian subset of the Daytracker Study, an investigation of the relationship between well-being and health in working women. Only women were included in this study, as there are very few studies that address the population of working women [15]. The study was approved by the Research Ethics Committees of Semmelweis University and University College London, and all participants signed an informed consent form. Participants received a small honorarium at the end of the study.
2.1. Participants
Participants were recruited from full-time female employees of Semmelweis University in Budapest via emails and flyers. The inclusion criteria were explicitly declared during recruitment, thus volunteers applied only if not 1) pregnant; 2) suffering from acute or chronic illness such as cardiovascular disease, diabetes, cancer, and endocrine disorder; 3) diagnosed with mental disorder (e.g. major depression, PTSD, and bipolar disorder); 4) taking steroid, hypertensive or anti-inflammatory medication or beta-blockers. From the initial 202 included participants, we excluded those with missing data on nightmares (N = 6), and morning cortisol level (N = 8). Thus, the final size of the study sample was 188.
2.2. Procedure and assessment
After a briefing about the study protocol, participants completed two 24 h assessments on a working day and on a leisure day. The 24-hour periods started at 17 h and ended next day. To avoid any sequence effects, assessment randomly started on a working or a leisure day. During the assessments, participants provided seven saliva samples each day — using Salivettes (Sarstedt, Leicester, UK). Participants were instructed not to eat, drink or brush their teeth until after the 30 min postwaking sample, and 15 min before later saliva samplings. Saliva samples were timed immediately at waking, 30 min after awakening, at 10 h, 12 h, 15 h, 17 h, and at bedtime. Saliva samples were stored in a cold place or refrigerator until they were transported to the university lab within 1–2 days. Subsequently, the samples were kept frozen at −20 °C until analysis. Analysis was carried out using a high sensitivity chemiluminescence assay at the Technical University in Dresden (Germany). Inter- and intra-assay coefficients of variance (CVs) were b 8%. CAR was calculated using the area-under-the-curve with respect to ground (AUC G), using the waking and the 30 min post-awakening cortisol values [16]. We chose this method as it is relatively robust and after natural log transformation it followed normal distribution (Shapiro–Wilk normality tests: W = 0.99, p = .520, and W = 0.99, p = .423 for working and leisure day CAR, respectively). Standardized survey questions and questionnaires were used to assess demographic data, mental and somatic health, lifestyle, and sleep quality [see 17]. These factors were used as covariates to exclude known confounders of the CAR [16]. Tests relevant to the current study included depressive symptoms (CES-D, Cronbach’s α = .88), trait anxiety (STAI-T, α = .92), perceived health (PHQ-15, α = .80), Jenkins sleep problem questionnaire (α = .75), and morningness– eveningness scale (α = .89). The items of these questionnaires were rated on four- or five-point Likert scales. Participants also answered single questions – that were dichotomized later – about alcohol consumption (non-drinker/drinker), smoking status (non-smoker/smoker), and number of children. Stress was assessed using ecological momentary assessment (EMA) [18], whereby participants answered the question “On a scale of 1 to 5, please rate how stressed you are at this moment” seven times over one workday and leisure day at the same time when saliva samples were taken (see below). Mean stress scores were calculated for each day. Participants were asked if they had nightmares frequently using a single binary question: “Do you frequently have nightmares that wake you up?”. This measure has been used commonly in nightmare studies [2]. Moreover, participants reported the emotional quality of dreams on days of the measurement using single choice questions (“How would you describe the emotional quality of your dreams?”; options: very unpleasant, unpleasant, neutral, pleasant, very pleasant, do not remember).
2.3. Statistical analysis
Welch’s t-tests and chi-square tests were used to compare the characteristics of the nightmare and non-nightmare groups. To investigate the effect of frequent nightmares on CAR, we carried out separate ANCOVAs on working and leisure days controlling for age, BMI, morningness, education, depression, anxiety, physical symptoms, sleep quality, alcohol consumption, smoking, physical exercise, and sleeping time. Data analysis was performed with R 3.1.2 [19].
- Results
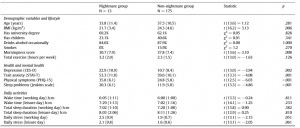
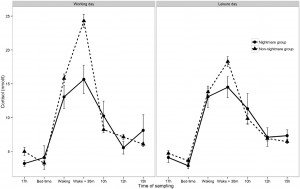
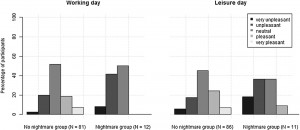
Preliminary analysis showed that participants with frequent nightmares had a significantly lower BMI, were more depressed and anxious, reported more sleep problems and somatic symptoms, and showed a lower morningness score. The frequent nightmare group also reported more stress than participants without frequent nightmares on both days, although this difference was on the threshold of statistical significance. Differences were not significant for sleep duration, demographic, and lifestyle related variables (see Table 1). Fig. 1 shows the profiles of cortisol output over the work and leisure days. We found typical diurnal profiles, with relatively high cortisol on waking, an increase over the first 30 min of the day, and progressive decreases in output across the day. Those participants who experienced nightmares frequently showed a significantly smaller CAR than those who did not experience nightmares frequently F (1,186) = 6.98, p = .009, η2p = .04 (values without covariates). The relationship remained significant after controlling for all covariates (F (1,153) = 4.72, p = .030, η2p = .026), however due to the list-wise deletion of missing values, the sample size changed to 155 (nightmare group = 11, nonnightmare group = 144). Moreover, given that the inclusion of covariates only controls for linear relationships between the outcome and the predictors, we conducted an additional analysis on a subsample of non-nightmare sufferers matched to the subgroup of nightmare sufferers by age, BMI, sleep quality (Jenkins score), and morningness scores. The difference in CAR between the nightmare group and the matched control was significant on the working day F (1,24) = 4.30, p = .049, η2p = .15 (for details of the matched sample, please refer to the online supplement). The difference in CAR between the nightmare and non-nightmare groups was not significant on the leisure day F (1,183) = 0.78, p = .379, η 2p = .004. There were no significant differences between the two groups at the other time points of either day (ts b 1.53, ps N .15). Moreover, the working day CAR and the leisure day CAR of the nightmare group were not different t(12) = −0.06, p = .952, while in the non-nightmare group the CAR was significantly higher on the working day than on the leisure day t(171) = 6.77, p b .001. Significantly more participants could recall the emotional quality of their dreams in the nightmare group than in the non-nightmare group on the working day (92% vs. 48%, respectively; χ2 (1) = 7.71, N = 181, p = .005) as well as on the leisure day (84% vs. 51%, respectively; χ2 (1) = 4.33, N = 183, p = .037). The emotional quality of dreams was not significantly different between the groups on either day (working day χ2 (4) = 6.45, N = 95, p = .168; leisure day (χ2 (4) = 5.81, N = 99, p = .214). Fig. 2 shows the percentage of participants reporting a particular emotional quality of dreams on the two days.
Table 1 Differences between participants with and without frequent nightmares.
Note: Values represent means (SD) or percentages. Statistics were calculated using Welch’s t tests and Pearson’s Chi-square tests with Yates continuity correction.
The emotional quality of the actual morning’s dream report was not associated with CAR (working day: F (4,88) = 0.61, p = .659; leisure day: F (4,90) = 1.35, p = .258). Recall of a bad dream on the day of the measurement added to the model as a dichotomous covariate (0: neutral or pleasant dream, 1: unpleasant and very unpleasant dream) was not significant (working day: F (1,90) = 0.67, p = .414; leisure day: F (1,92) = 0.04, p = .852).
- Discussion
In a non-clinical sample of working women, we found that frequent nightmares were associated with a blunted CAR on a working day. This finding was independent of age, BMI, education, depression, anxiety, sleep time and quality, chronotype, physical symptoms, and health behaviors (physical activity, smoking, and alcohol consumption). To the best of our knowledge, this is the first study to demonstrate an association between CAR and the condition of experiencing nightmares frequently. In coherence with earlier studies, participants with frequent nightmares rated themselves as more depressed, anxious, were characterized by more sleep problems and lower morningness score, and experienced increased daily stress [2,3,20]. This co-morbidity of psychological complaints and nightmares is often attributed to heightened emotional reactivity [3].
Fig. 1. Diurnal cortisol levels of women having frequent nightmares (N = 13), and without frequent nightmares (N = 175) on a working and a leisure day. Data points represent untransformed values, error bars represent SEM.
Fig. 2. Percentage of participants reporting a particular emotional quality on the days of the assessment.
One possible function of the CAR is to facilitate coping with the upcoming daily stresses [21]. Accordingly, the present study has shown that participants without frequent nightmares had a smaller CAR on the leisure day compared to the working day. Similar findings have been reported in other studies [22]. Interestingly, participants with frequent nightmares did not show a CAR difference between the two days. We might speculate that nightmare sufferers have a limited physiological adaptability to anticipated stressors. Nightmare sufferers recalled more dreams during the study period, however, flattened CAR was not related to the emotional quality of reported dreams, regardless the type of the day (working or leisure). This suggests, that reduced CAR in the nightmare group during the working days reflects a trait-like vulnerability of the HPA axis.
This finding argues against a state-like dependency between CAR and negative dream experiences and is in coherence with earlier findings showing altered physiological sleep parameters in nightmare sufferers regardless of having nightmares on the time of the measurement [23,24]. As previous studies reported, nightmare disorder is usually associated with poor sleep quality, including subjective reports and objective sleep indices [11,25,23]. It would be plausible to assume that spontaneous, brief awakenings or hyperarousal during sleep (previously reported among nightmare sufferers [23]) might also increase nocturnal cortisol secretion and lead to attenuated CAR through the exhaustion of HPA reactivity [16,26]. To investigate this option, Dettenborn and her colleagues studied the CAR after waking participants up several times during three consecutive nights [27]. They found no evidence for the presumed effect of the repeated forced awakenings on the morning CAR. However, it is possible that the exhaustion of the CAR develops only in the long term. This possibility is also supported by the findings of the present study, whereby bad dreams on the measurement day were not associated with diminished CAR while frequent nightmares were. Persisting alterations in HPA function might reflect structural changes, as in the case of PTSD [28].
Our findings of blunted CAR in subjects reporting frequent nightmares, resemble the data regarding the association between PTSD symptoms and flattened CAR [2,12–14]. Atypical HPA axis activation, sleep disturbances and frequent nightmares are prevalent features of PTSD [29]. Increased arousal-promoting neural activity reflected by increased noradrenergic tone during sleep seems to contribute to poor sleep quality and nightmarish experiences in these patients [30]. Our data suggests that an association between blunted CAR and frequent nightmares might be present among non-clinical populations as well. However, the clinical relevance of this association will need to be addressed by further studies. Frequent nightmares may be the result of dysfunctional autonomic regulation in sleep. Nielsen and Levin [8] proposed that during dreaming, distressing memories are reconstructed, recombined, and recontextualized in order to change the associated emotions, eventually leading to the extinction of fearful memories. An important element during this process is the desynchronization of the emotional content and the autonomic reaction in REM sleep (i.e. an increased emotional distress coupled with low autonomic response). During nightmares, this fear extinction mechanism is supposed to be impaired, because the autonomic response in REM sleep is too intense [2,8]. Frequent nightmares might reflect a recurring dysfunction in the autonomic regulation during the REM phase. It may be also possible that this autonomic arousal is reflected in the HPA response. Unfortunately, we are not aware of any studies that measured HPA activity during actual nightmares.
The present findings might serve as a preliminary indication that the HPA system is involved in the production of nightmares. It is important to emphasize that measures were taken in a general population sample that was not primed to the study of nightmares. Another strength of our study is that we measured psychosocial factors with standard questionnaires, and employed EMA to assess stress over the day. The limitations of the study should also be noted. Categorization of participants to nightmare and control groups was done by using a single question. Although this method is prevalent in the literature, it can limit the findings [2]. As nightmares, by definition, have a negative influence on sleep quality, it is difficult to completely rule out the possibility that the blunted CAR was the result of poor sleep and not the nightmares per se – even though this possibility was accounted for statistically. Moreover, the study involved university-based working women which may limit the generalizability of the results. Further, the effect size for the CAR difference between the nightmare and nonnightmare groups on the working day is modest, and studies with larger group sizes are needed to verify the association of frequent nightmares and diminished CAR.
- Conclusion
The association between blunted CAR suggests altered HPA functioning in nightmare sufferers. This altered functioning might result from reduced physiological adaptability to stressors in individuals with frequent nightmares. Further studies are needed to explore the association between the HPA system and the production of nightmares.
Acknowledgements
This study was supported by the Economic and Social Research Council and National Institute of Aging [RES-177-25-0005; AG13196]. We are grateful to Clemens Kirschbaum, Technical University Dresden, for carrying out the cortisol assays.
Appendix A Supplementary data
Supplementary data to this article can be found online at http://dx. doi.org/10.1016/j.physbeh.2015.05.001.
References
[1] V.I. Spoormaker, M. Schredl, J. van den Bout, Nightmares: from anxiety symptom to sleep disorder, Sleep Med. Rev. 10 (2006) 19–31, http://dx.doi.org/10.1016/j. smrv.2005.06.001.
[2] R. Levin, T.A. Nielsen, Disturbed dreaming, posttraumatic stress disorder, and affect distress: a review and neurocognitive model, Psychol. Bull. 133 (2007) 482–528, http://dx.doi.org/10.1037/0033-2909.133.3.482.
[3] M. Schredl, Effects of state and trait factors on nightmare frequency, Eur. Arch. Psychiatry Clin. Neurosci. 253 (2003) 241–247, http://dx.doi.org/10.1007/s00406-0030438-1.
[4] M. Schredl, Nightmares as a paradigm for studying the effects of stressors, Sleep 36 (2013) 969–970, http://dx.doi.org/10.5665/sleep.2784.
[5] S. Van Liempt, M. Van Zuiden, H. Westenberg, A. Super, E. Vermetten, Impact of impaired sleep on the development of PTSD symptoms in combat veterans: a prospective longitudinal cohort study, Depress. Anxiety. 30 (2013) 469–474, http://dx.doi. org/10.1002/da.22054.
[6] F.L. Coolidge, D.L. Segal, C.M. Coolidge, F.M. Spinath, J. Gottschling, Do nightmares and generalized anxiety disorder in childhood and adolescence have a common genetic origin? Behav. Genet. 40 (2010) 349–356, http://dx.doi.org/10.1007/s10519009-9310-z. [7] P. Simor, P. Pajkossy, K. Horváth, R. Bódizs, Impaired executive functions in subjects with frequent nightmares as reflected by performance in different neuropsychological tasks, Brain Cogn. 78 (2012) 274–283, http://dx.doi.org/10.1016/j.bandc.2012. 01.006.
[8] T. Nielsen, R. Levin, Nightmares: a new neurocognitive model, Sleep Med. Rev. 11 (2007) 295–310, http://dx.doi.org/10.1016/j.smrv.2007.03.004.
[9] J.P. Herman, M.M. Ostrander, N.K. Mueller, H. Figueiredo, Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis, Prog. Neuro-Psychopharmacol. Biol. Psychiatry 29 (2005) 1201–1213, http://dx.doi. org/10.1016/j.pnpbp.2005.08.006.
[10] J. Backhaus, K. Junghanns, F. Hohagen, Sleep disturbances are correlated with decreased morning awakening salivary cortisol, Psychoneuroendocrinology 29 (2004) 1184–1191, http://dx.doi.org/10.1016/j.psyneuen.2004.01.010.
[11] P. Simor, K. Horváth, F. Gombos, K.P. Takács, R. Bódizs, Disturbed dreaming and sleep quality: altered sleep architecture in subjects with frequent nightmares, Eur. Arch. Psychiatry Clin. Neurosci. 262 (2012) 687–696, http://dx.doi.org/10.1007/ s00406-012-0318-7.
[12] N. Rohleder, L. Joksimovic, J.M. Wolf, C. Kirschbaum, Hypocortisolism and increased glucocorticoid sensitivity of pro-inflammatory cytokine production in Bosnian war refugees with posttraumatic stress disorder, Biol. Psychiatry 55 (2004) 745–751, http://dx.doi.org/10.1016/j.biopsych.2003.11.018.
[13] C.S. de Kloet, E. Vermetten, C.J. Heijnen, E. Geuze, E.G.W.M. Lentjes, H.G.M. Westenberg, Enhanced cortisol suppression in response to dexamethasone administration in traumatized veterans with and without posttraumatic stress disorder, Psychoneuroendocrinology 32 (2007) 215–226, http://dx.doi.org/10.1016/j. psyneuen.2006.12.009.
[14] M. Wessa, N. Rohleder, C. Kirschbaum, H. Flor, Altered cortisol awakening response in posttraumatic stress disorder, Psychoneuroendocrinology 31 (2006) 209–215, http://dx.doi.org/10.1016/j.psyneuen.2005.06.010.
[15] A. Steptoe, J. Wardle, A. Stone, D. Kahneman, M. Marmot, International Study of Biology and Positive Well-being: Full Research Report ESRC End of Award Report, RES-177-25-0005, ESRC, Swindon, 2009.http://dx.doi.org/10.5255/UKDA-SN-66921.
[16] Y. Chida, A. Steptoe, Cortisol awakening response and psychosocial factors: a systematic review and meta-analysis, Biol. Psychol. 80 (2009) 265–278, http://dx.doi. org/10.1016/j.biopsycho.2008.10.004.
[17] R.Á. Haraszti, G. Purebl, G. Salavecz, L. Poole, S. Dockray, A. Steptoe, Morningness– eveningness interferes with perceived health, physical activity, diet and stress levels in working women: a cross-sectional study, Chronobiol. Int. (2014) 1–9, http://dx. doi.org/10.3109/07420528.2014.911188.
[18] S. Shiffman, A. Stone, M.R. Hufford, Ecological momentary assessment, Annu. Rev. Clin. Psychol. 4 (2008) 1–32, http://dx.doi.org/10.1146/annurev.clinpsy.3.022806. 091415.
[19] R Core Team, R: A Language and Environment for Statistical Computing, 2014.
[20] T. Nielsen, Nightmares associated with the eveningness chronotype, J. Biol. Rhythm. 25 (2010) 53–62, http://dx.doi.org/10.1177/0748730409351677.
[21] E. Fries, L. Dettenborn, C. Kirschbaum, The cortisol awakening response (CAR): facts and future directions, Int. J. Psychophysiol. 72 (2009) 67–73, http://dx.doi.org/10. 1016/j.ijpsycho.2008.03.014.
[22] S.R. Kunz-Ebrecht, C. Kirschbaum, M. Marmot, A. Steptoe, Differences in cortisol awakening response on work days and weekends in women and men from the Whitehall II cohort, Psychoneuroendocrinology 29 (2004) 516–528, http://dx.doi. org/10.1016/S0306-4530(03)00072-6.
[23] P. Simor, R. Bódizs, K. Horváth, R. Ferri, Disturbed dreaming and the instability of sleep: altered nonrapid eye movement sleep microstructure in individuals with frequent nightmares as revealed by the cyclic alternating pattern, Sleep 36 (2013) 413–419, http://dx.doi.org/10.5665/sleep.2462.
[24] P. Simor, J. Körmendi, K. Horváth, F. Gombos, P.P. Ujma, R. Bódizs, Electroencephalographic and autonomic alterations in subjects with frequent nightmares during preand post-REM periods, Brain Cogn. 91 (2014) 62–70, http://dx.doi.org/10.1016/j. bandc.2014.08.004.
[25] A. Steiger, Sleep and the hypothalamo-pituitary-adrenocortical system, Sleep Med. Rev. 6 (2002) 125–138, http://dx.doi.org/10.1053/smrv.2001.0159.
[26] I. Wilhelm, J. Born, B.M. Kudielka, W. Schlotz, S. Wüst, Is the cortisol awakening rise a response to awakening? Psychoneuroendocrinology 32 (2007) 358–366, http:// dx.doi.org/10.1016/j.psyneuen.2007.01.008.
[27] L. Dettenborn, F. Rosenloecher, C. Kirschbaum, No effects of repeated forced wakings during three consecutive nights on morning cortisol awakening responses (CAR): a preliminary study, Psychoneuroendocrinology 32 (2007) 915–921, http://dx.doi. org/10.1016/j.psyneuen.2007.06.011.
[28] G. Villarreal, D.A. Hamilton, H. Petropoulos, I. Driscoll, L.M. Rowland, J.A. Griego, et al., Reduced hippocampal volume and total white matter volume in posttraumatic stress disorder, Biol. Psychiatry 52 (2002) 119–125, http://dx.doi.org/10.1016/ S0006-3223(02)01359-8.
[29] A. Germain, D.J. Buysse, E. Nofzinger, Sleep-specific mechanisms underlying posttraumatic stress disorder: integrative review and neurobiological hypotheses, Sleep Med. Rev. 12 (2008) 185–195, http://dx.doi.org/10.1016/j.smrv.2007.09.003.
[30] M. Ahmadpanah, P. Sabzeiee, S.M. Hosseini, S. Torabian, M. Haghighi, L. Jahangard, et al., Comparing the effect of prazosin and hydroxyzine on sleep quality in patients suffering from posttraumatic stress disorder, Neuropsychobiology 69 (2014) 235–242, http://dx.doi.org/10.1159/000362243.