SLEEP 2013;36(3):413-419.
DOI: 10.5665/sleep.2462
Authors
Péter Simor, Msc1; Róbert Bódizs, PhD2; Klára Horváth, MD3; Raffaele Ferri, MD4
Affiliations
1 Department of Cognitive Sciences, Budapest University of Technology and Economics, Budapest, Hungary;
2 Institute of Behavioural Sciences, Semmelweis University, Budapest, Hungary;
3 Department of Experimental Psychology, University of Oxford, United Kingdom;
4 Department of Neurology I.C. Sleep Research Centre, Oasi Institute for Research on Mental Retardation and Brain Aging (IRCCS), Troina, Italy 1
Address correspondence to: Péter Simor, Msc, H-1111 Egry József u. 1. Tépület./V.em. Budapest, Hungary; Tel: +36 1 463-1273; Fax: +36 1 4631072; E-mail: petersimor@gmail.com or psimor@cogsci.bme.hu
Abstract
Study Objectives: Nightmares are disturbing mental experiences during sleep that usually result in abrupt awakenings. Frequent nightmares are associated with poor subjective sleep quality, and recent polysomnographic data suggest that nightmare sufferers exhibit impaired sleep continuity during nonrapid eye movement (NREM) sleep. Because disrupted sleep might be related to abnormal arousal processes, the goal of this study was to examine polysomnographic arousal-related activities in a group of nightmare sufferers and a healthy control group.
Design: Sleep microstructure analysis was carried out by scoring the cyclic alternating pattern (CAP) in NREM sleep and the arousal index in rapid eye movement (REM) sleep on the second night of the polysomnographic examination.
Setting: Hospital-based sleep research laboratory.
Participants: There were 17 in the nightmare (NMs) group and 23 in the healthy control (CTLs) group.
Interventions: N/A. Measurements and Results: The NMs group exhibited reduced amounts of CAP A1 subtype and increased CAP A2 and A3 subtypes, as well as longer duration of CAP A phases in comparison with CTLs. Moreover, these differences remained significant after controlling for the confounding factors of anxious and depressive symptoms. The absolute number and frequency of REM arousals did not differ significantly between the two groups.
Conclusions: The results of our study indicate that NREM sleep microstructure is altered during nonsymptomatic nights of nightmares. Disrupted sleep in the NMs group seems to be related to abnormal arousal processes, specifically an imbalance in sleep-promoting and arousing mechanisms during sleep.
Keywords: Arousals, cyclic alternating pattern (CAP), dreaming, nightmare, sleep microstructure
INTRODUCTION
According to the International Classification of Sleep Disorders,1 nightmares are disturbing mental experiences that often awaken the dreamer from late-night rapid eye movement (REM) sleep. The co-occurrence of disturbed dreaming and psychopathologic symptoms2 has contributed to the assumption that nightmares are the secondary symptom of an underlying mental disorder. Although comorbid psychopathology may increase the severity and daytime effects of disturbed dreaming, research suggests that frequent nightmares should be considered as a specific sleep disorder that are independent in its origins from other mental complaints.3-6 A growing body of research indicates that nightmares are related to impaired subjective sleep quality in large cohorts of adults, children, and university students.7-9 Furthermore, negatively toned dreams are more frequent among individuals with insomnia and other sleep disordered persons,10,11 and nightmare frequency seems to be related to the severity of sleep complaints.10,12 Early sleep electroencephalography (EEG) studies revealed altered sleep architecture in idiopathic nightmare sufferers reflected by decreased total sleep time and slow-wave sleep (SWS), as well as an increase in nocturnal awakenings.13,14 A later study15 with a relatively small sample size failed to confirm these findings but reported enhanced motor activation in NREM and REM sleep in idiopathic and posttraumatic nightmare sufferers, suggesting that increased arousal and the release of motor inhibition are related to negative dream experiences. Another study16 reported heightened sympathetic arousal in nightmare sufferers during NREM and REM periods after a partial REM deprivation procedure. A more recent polysomnographic study 17 found that nightmare sufferers, in comparison with control patients, exhibited reduced sleep efficiency, increased wakefulness after sleep onset, decreased SWS, and higher number of nocturnal awakenings, especially from Stage 2 sleep. Nightmare sufferers also showed increased duration of REM sleep that was mediated by questionnaire measures of heightened daytime negative affect. It is important to note that altered sleep architecture was not related to the occurrence of nightmares in these studies. In fact, because nightmares have almost never been reported in laboratory settings even in frequent nightmare sufferers, these findings indicate that impaired sleep regulation is an inherent, presumably traitlike feature of nightmare sufferers’ sleep pathophysiology. Nightmares are assumed to arise mainly from REM sleep5; however, Simor and colleagues17 showed that sleep instability in nightmare sufferers is more pronounced in NREM sleep. Impaired NREM sleep continuity, frequent awakenings from Stage 2 sleep, decreased SWS, and enhanced motor and sympathetic activation may reflect dysfunctional arousal processes, an imbalance of sleep-promoting and arousing mechanisms during sleep. Cyclic alternating pattern (CAP)18,19 is a spontaneous rhythm of NREM sleep characterized by EEG oscillations corresponding to recurrent activation events and unstable sleep depth. Since its discovery,19 CAP has been extensively applied to the study of human sleep, in normal and pathologic conditions, in a wide age range from newborns to adults and elderly,18,20 and is now considered to be a comprehensive tool to study sleep instability.18 The analysis of CAP has already been shown to be useful in the understanding of neurophysiologic mechanisms of different NREM sleep parasomnia in children20-22 and also in REM sleep parasomnia of adults.23,24 Thus, we considered that the fine grained microstructural analysis examining the nature of arousals in NREM sleep that can be carried out by CAP analysis would shed more light on the anomalies of sleep-wake regulation of nightmare sufferers. Moreover, to verify whether abnormal arousal processes are specific to NREM sleep or are also present in REM sleep, we analyzed arousal processes in REM sleep as well. We expected increased NREM sleep instability in nightmare sufferers, reflected by enhanced CAP rate, especially of the subtypes A2 and A3. Furthermore, based on the independence of nightmare-related NREM alterations from daytime distress,17 we hypothesized that increased arousal processes during NREM sleep would be independent of the confounding effects of waking anxiety and/or depressive symptoms. Based on previous findings, we also hypothesized increased microarousals in nightmare sufferers during REM sleep, but we expected this enhancement to be mediated by waking psychopathological symptoms.
METHODS
Participants
Participants (all native Hungarians) were selected from a large pool of undergraduate students from the Budapest University of Technology and Economics and the Semmelweis University. The selection procedure of nightmare sufferers (NMs) and a control group (CTLs) was described previously in detail.17 In brief, individuals were selected based on their scores on three different dreaming-related questionnaires: the Dream Quality Questionnaire (DQQ),25 the Hungarian version of the Van Dream Anxiety Scale (VDAS-H),26 and a 7-point Likert scale assessing the frequency of nightmares. Individuals reporting one or more nightmares per week in the retrospective questionnaires were assigned to the NMs group, whereas individuals having fewer than two nightmares during the past year were assigned as CTLs. Those individuals who reported the onset of negative dream experiences in relation to a traumatic event or indicated that the content of their dreams was related to a previous trauma (such as physical attack, accident, sudden death of a close relative, etc.) were excluded from the study. Finally, 17 NMs (7 females and 10 males) (Mage = 20.65 ± 1.73) and 23 CTLs (12 females and 11 males) (Mage = 21.35 ± 1.61) underwent polysomnographic studies. (The difference in age was not significant between the two groups: U(38) = 134; Z = -1.719; P = 0.086). NMs scored higher on the Negative Dream Affect Scale of the DQQ (MNMs = 7.8; SDNMs = 1.81 versus MCTLs = 4.04; SDCTLs = 1.69; t(38) = -6.83; P < 0.001) and on the VDAS-H (MNMs = 19.53; SDNMs = 7.21 versus MCTLs = 0.26; SDCTLs = 0.62; t(16.17) = -10.99; P < 0.001; equal variances not assumed), indicating at least moderately severe dream disturbances.25,26
None of the individuals reported previous neurological, psychiatric, or sleep disorders or history of any chronic disease. The study protocol was approved by the Ethical Committee of the Semmelweis University. The individuals received monetary compensation for their participation in the sleep laboratory investigations. Written informed consent was obtained.
Psychometric Tests
The State-Trait Anxiety Inventory (STAI)27 is a widely used self-report instrument that differentiates the temporary condition of state anxiety and the long-standing quality of trait anxiety.
We used the 20-item Hungarian version of the STAI-T (Trait Anxiety) to assess general levels of anxiety.28 We used the short form of the Hungarian version of the Beck Depression Inventory (BDI-H)29 to measure the extent of waking depressive symptoms in our study participants. The 9-item BDI-H is a one-dimensional scale assessing different symptoms of depression including social withdrawal, indecision, sleep disturbance, fatigue, intense worry about bodily symptoms, work inhibition, pessimism, lack of satisfaction, and self-accusation.
The Hungarian version of the Groningen Sleep Quality Scale (GSQS) was used for measuring subjective sleep quality.30 The 14-item questionnaire measures the extent of subjective sleep fragmentation by a binary scale.
Procedure
Polysomnographic recordings were performed in the sleep research laboratory of the Semmelweis University for 2 consecutive nights. (The first night served as the adaptation night). Study participants were not allowed to drink alcohol or take drugs (except contraceptives) on the day of and the day before the examination. They were asked to avoid napping and consuming caffeine on the afternoon of the sleep recordings. Study participants completed the short version of the BDI-H upon arrival. (The STAI-T was completed previously during the selection procedure.) The timing of lights off was between 11:00 and 01:00 depending on each participant’s preferred bedtime. Morning awakenings were scheduled after 9 hours of undisturbed sleep except if participants woke up earlier spontaneously. In the morning, participants were asked to complete the GSQS and to report their dreams. Three of the NMs reported negatively toned dreams in the laboratory.
Polysomnography
During the standard polysomnographic examination on both nights, participants were fitted with 19 EEG electrodes (Fp1, Fp2, F3, F4, Fz, F7, F8, C3, C4, Cz, P3, P4, Pz, T3, T4, T5, T6, O1, O2) according to the 10-20 electrode placement system31 as well as with two electrodes (bipolar channel) monitoring vertical and horizontal eye movements; electromyography (EMG) electrodes (bipolar channels) for the chin and for the anterior tibialis muscles, 2 ECG electrodes according to standard lead I; in addition to the thoracic and abdominal respiration sensors. Gold-coated Ag/ AgCl EEG cup electrodes were fixed with EC2 Grass Electrode Cream (Grass Technologies, West Warwick, USA) and referred to the mathematically-linked mastoids. Impedances were kept below 8 kΩ. Signals were collected, prefiltered (0.33-1,500 Hz, 40dB/decade anti-aliasing hardware input filter), amplified, and digitized with 4,096 Hz/channel sampling rate (synchronous) with 12-bit resolution by using the 32-channel EEG/polysystem (Brain-Quick BQ 132S, Micromed, Treviso, Italy). A further 40 dB/decade antialiasing digital filter was applied by digital signal processing, which low-pass filtered the data at 450 Hz. Finally, the digitized and filtered EEG was undersampled at 1,024 Hz.
CAP Scoring
Sleep stages and conventional measures of sleep macrostructure were previously scored according to standard criteria32 by two experienced sleep researchers who were blind to the group membership of the participants. CAP was scored according to the criteria published by Terzano et al.33 CAP is a periodic EEG activity of NREM sleep characterized by sequences of cycles composed of a phase A (transient electrocortical event) and a phase B (recurring EEG background activity). Phase A activities can be classified into three subtypes. This classification is based on the reciprocal proportion of high-voltage slow waves (EEG synchrony) and low-amplitude fast rhythms (EEG desynchrony). Subtype A1 shows a predominance of synchronized EEG activity; if present, EEG desynchrony occupies less than 20% of the entire A phase duration. Subtype A1 specimens include delta bursts, K-complex sequences, vertex waves, and polyphasic bursts with less than 20% of EEG desynchrony. Subtype A2 is scored in the presence of 20-50% of desynchronized EEG activity, with predominance of polyphasic bursts. Subtype A3 EEG activity is predominantly rapid low-voltage rhythms with more than 50% of phase A occupied by EEG desynchrony. Subtype A3 include EEG arousals and K-alpha and polyphasic bursts (with at least 50% of EEG desynchrony). CAP cycles are based on the presence of two successive phases A and B. CAP sequences are defined as three or more A phases separated from each other by no more than 60 s. CAP rate is defined as the percentage of total NREM time occupied by CAP sequences. The remaining NREM sleep is called non-CAP (NCAP). All CAP events were visually detected and marked on recordings and CAP parameters (CAP rate, rate and duration of CAP A subtypes, duration of A and B phases as well as the frequency (number/h) of A subtypes: A1, A2, A3 index) were extracted by means of the sleep analysis software Hypnolab 1.2 (SWS Soft, Italy).
REM Arousals
Arousals during REM sleep were detected visually according to the criteria of the American Sleep Disorders Association34 by a blind rater trained on sleep arousal scoring. REM arousals were scored as abrupt increases of EEG frequencies including theta, alpha, or beta activities for at least 3 sec as well as an increase (for at least 1 sec) of EMG activity in chin muscle tone. The absolute number of REM arousals as well as the frequency of REM arousals (the REM arousal index: the number of REM arousals/h) was used for statistical analyses.
Statistical Analyses
Statistical analyses were carried out with the Statistical Package for the Social Sciences (SPSS) version 19.0. Mean scores of the psychometric tests were compared with independent samples t test; if the criterion for homogeneity of variance was violated, we applied the Welch test. For those psychometric variables that were not normally distributed (according to the Shapiro-Wilk test), the Mann-Whitney U test was used. Those variables that were characterized by positively skewed distributions were transformed to a natural logarithmic scale to normalize their distribution. CAP variables as dependent factors were entered into a multivariate analysis of variance (MANOVA) model with group membership as an independent factor. To control for the confounding effects of daytime waking distress, we carried out a multivariate analysis of covariance (MANCOVA) with the same dependent and independent factors as well as the BDI-H and STAI-T scores as covariates in the model. Similar analyses were carried out for REM arousals. Significance level was set at P < 0.05 and the Bonferroni corrected value (P < 0.005) was considered in case of multiple comparisons of different independent variables.
RESULTS
Psychometric Variables and Sleep Architecture
A detailed description of psychometric assessments and differences regarding standard sleep architecture has been previously reported elsewhere.17 Nonetheless, for clarity we provide a brief summary of these results. NMs in comparison with CTLs were characterized by significantly higher scores on the STAI-T and BDI-H questionnaires, indicating mild anxiety and depressive symptoms. NMs and CTLs did not significantly differ regarding subjective sleep fragmentation. In contrast, objective sleep measures showed increased wakefulness and Stage 1 sleep, as well as decreased SWS, reduced sleep efficiency, and more nocturnal awakenings in NMs. NMs also showed increased REM sleep; however, this was mediated by heightened waking affect measured by the psychometric assessments.
CAP Analysis
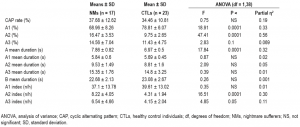
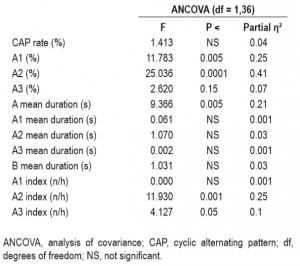
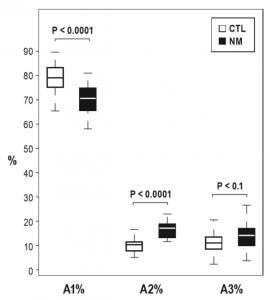
The comparison of CAP parameters with MANOVA revealed a significant main effect for Group (F(12,27) = 5.53; P < 0.001; Partial η2 = 0.71), indicating that NMs and CTLs show different patterns regarding the microstructure of NREM sleep. Descriptive statistics and univariate tests of CAP indices are summarized in Table 1. Although the overall CAP rate was similar, the distributions of CAP A subtypes showed marked differences between the two groups (Figure 1). NMs exhibited a significantly lower percentage of A1 and higher rates of A2 as well as a trend of increase in the number of A3 subtypes. If the time spent in NREM sleep was controlled, the increase of A2 subtype (A2 index) remained significant, the increase in A3 index showed a trend, but the decrease in A1 index was not significant. Apart from these differences, the mean duration of CAP A phases was significantly longer in NMs in comparison with the CTLs. The comparison of the other CAP parameters did not reveal significant differences (Table 1). To control for the confounding effects of waking anxiety and depressive symptoms, we carried out a MANCOVA with STAIT and BDI-H scores as covariates in the model. The difference between NMs and CTLs remained significant (F(12,25) = 3.3; P = 0.006; partial η2 = 0.61). In accordance with the previous analysis, significant group differences emerged for A1% and A2% as well as a weak trend for A3%. A2 index and the mean duration of CAP A phases remained significantly increased in NMs in comparison with CTLs, and the increase of A3 index in NMs showed a trend (Table 2).
REM Arousals
A main effect for Group emerged F(2,37) = 3.93; P = 0.028; partial η2 = 0.18 between NMs and CTLs; however, neither the number (MNMs = 38.82; SDNMs = 17.21 vs. MCLTs = 33.43;
Table 1—Comparison of CAP variables between NMs and CTLs
Table 2—Analysis of covariance group (nightmare sufferers, healthy control individuals) effects with STAI-T (STAI-Trait Anxiety), Beck Depression Inventory (Hungarian version) as covariates.
Figure 1—Group differences in the CAP subtypes: A1%, A2%, and A3%. Nightmare sufferers (NMs) show a significant decrease in A1% and a significant increase in A2% compared with healthy control individuals (CTLs).
SDCTLs = 15.67; F(1,38) = 1.06; P = 0.31) nor the frequency of REM arousals (REM arousal index) (MNMs = 21.71; SDNMs = 7.79 versus MCLTs = 23.2; SDCTLs = 11.52; F(1,38) = 0.21; P = 0.65) differed significantly between the groups. Therefore, the significant main effect of Group in the MANOVA refers to the difference regarding the nonoverlapping part of the two dependent variables (number and frequency of REM arousals). Because the only non-overlapping factor between the number and the frequency of REM arousals is the duration of REM sleep, the significant main effect for Group likely reflects the difference in REM duration. This was supported by the fact that after including the relative duration of REM sleep as a covariate in the same model, the main effect of Group was not significant (F(2,36) = 0.17; P = 0.87). Accordingly, with the STAI-T and BDI-H scores as covariates in the model, the effect of Group was not significant (F(2,35) = 0.2; P = 0.82) either. Nevertheless, the independent effect of the BDI-H was significant for the number (F(3,36) = 5.19; P = 0.029) and showed a trend for the frequency of REM arousals (F (3,36) = 4.02; P = 0.053). STAI-T showed a trend for REM arousal index (F(3,36) = 3.54; P = 0.068), but not for the absolute number of REM arousals (F(3,36) = 1.72; P = 0.2).
DISCUSSION
The results of our study indicate that the sleep of NMs is characterized by altered NREM microstructure in comparison with that of CTLs. Although the overall rate of CAP A phases was similar in the two groups, NMs showed a decrease in the rate of A1 and an increase in the rate and relative amount (sleep time was also considered) of A2 and A3 subtypes, as well as of the mean duration of A phases. Moreover, these differences were independent of the confounding effects of anxious and depressive symptoms measured by the psychometric tests. These findings confirm that the CAP analysis is a more than adequate method to unravel microstructural alterations in NMs. The reduced absolute amount of A1 is consistent with decreased SWS in NMs, as A1 subtypes are more prominent in the descending phases of sleep. NMs also showed enhanced mean duration of CAP A phases that might be related to the increase in A2 and A3 in NMs, because the duration of these CAP subtypes was longer in comparison with the A1 subtype. The expression of A1 involves the synchronization of slow EEG activity especially in frontal areas,35 which seems to facilitate the maintenance of sleep structure in states of transient instability36 as well as the efficient offline information processing during sleep.35 A2 and even more A3 subtypes seem to be generated in posterior areas with less (A2) or virtually no involvement (A3) of frontal cortical generators and comprise increased fast EEG activity, including alpha and beta waves.37,38 Reduced amount of A1 and increased rate of A2 and A3 in NMs reflect dysfunctional sleep-wake regulation and the imbalance of sleep-promoting and arousing mechanisms during NREM sleep. This apparent shift toward arousal processes composed of desynchronized, low amplitude, and high frequency activities confirms that impaired sleep continuity in NMs is related to abnormal microarousal processes during sleep. Therefore, our findings complement earlier reports on poor sleep quality4 and increased sympathetic16 and motor activity in NMs.15 Our results partially resemble the findings by Parrino and colleagues39 reporting altered sleep microstructure, especially enhanced A2 subtypes in patients with paradoxical insomnia. Nevertheless, despite the increased number of A2 subtypes, insomnia and nightmare sufferers seem to show different patterns regarding CAP parameters. Although the overall CAP rate was increased in patients with insomnia, the rate of CAP A1 and A3 subtypes was not altered in comparison with that of control patients.39 In contrast, we found nonaltered overall CAP rate, but reduced A1 and slightly increased A3 subtypes in NMs, suggesting that not the frequency of CAP bursts per se but the cortical reactions in response to arousing stimuli are different in NMs. However, the partial overlap between disturbed dreaming and insomnia is in coherence with questionnaire-based findings reporting increased prevalence of nightmares among patients with insomnia.10,40
In addition to the maintenance of sleep structure, the generation of CAP A1 subtypes during sleep was related to nextday enhancements of waking performance in various cognitive tasks that require frontal lobe functions. In contrast, the rate of A2 and A3 subtypes was associated with reduced performance in attentional, memory, and executive tasks.41 Decreased amount of A1 and increased rate of A2 and A3 in NMs are in conjunction with a recent study showing impaired executive functions in a group of NMs.42 CAP A1 subtypes may be related to homeostatic, restorative properties of NREM sleep fostering the fine-tuning of neural networks that support offline information processing during sleep and cognitive functions during waking.43 Moreover, these slow oscillations reflect the “effort” of the cortex to preserve sleep continuity by the reinforcement of the thalamic-basal forebrain gate against arousing impulses. In contrast, microarousals with higher frequencies generated in posterior areas reflect the failure of this gating process, when instead of the propagation of synchronized slow oscillations from anterior areas to posterior sites the cortex is shifted toward a more alert brain state.18 In brief, our findings suggest that NMs are impaired in the buildup of slow synchronized activity that stabilizes and maintains deep sleep predominantly in the first part of the night. Moreover, NMs seem to be more prone to increased cortical activation in response to arousing stimuli. Contrary to our hypothesis, NMs did not show arousal increments during REM sleep, but anxiety and depression scores seemed to be weakly related to the rate of REM arousals. This finding is in conjunction with previous studies indicating that the intensity of REM sleep is a biologic marker of affective dysregulation in depression and other mood disorders.44,45 It is also consistent with the study by Simor and colleagues17 showing that impaired sleep continuity in NREM sleep is an inherent feature of nightmare disorder independently of the comorbid psychopathologic symptoms, whereas increased duration of REM sleep is mediated by these factors. Because we did not wake up our study participants during the night to collect dream reports, we cannot make direct inferences about the relationship between arousals and nightmare formation. In fact, only three of the 17 NMs reported that they experienced dysphoric dreams during the night, consistent with earlier reports on the attenuation of nightmares in laboratory settings.5 Disrupted sleep with reduced slow wave activity (especially in frontal areas) may have detrimental effects on cognitive functions relying on the prefrontal cortex,46 the most vulnerable structure to sleep loss.47,48 Because the prefrontal cortex has a crucial role in self-regulation, including the regulation of emotional responses,49-51 we may speculate that dysfunctional restorative processes in prefrontal structures may increase the risk of heightened negative affect during waking and presumably during dreaming.2 Nevertheless, despite our first results providing novel data on the neurophysiology of NREM sleep in NMs, the relationship between disrupted sleep and disturbed mental activity during sleep should be pursued by further investigations including experimental manipulations of sleep structure or mental activity. Examining a relatively homogeneous sample may reduce the influence of different confounding factors; however, we should be careful with generalizing our data to other age groups and populations. Due to the association between the distribution of different CAP subtypes and the performance in different neuropsychological tasks during waking,41 the assessment of different cognitive and affective functions in the morning would have provided more information regarding the relationship between disrupted sleep and waking dysfunctions in NMs. Despite these limitations, to the best of our knowledge this is the first study that provides empirical evidence on the relationship between nightmare disorder and altered sleep microstructure in NREM sleep, contributing to our understanding of the pathophysiology of disturbed dreaming.
Abbreviations
ANCOVA, analysis of covariance
ANOVA, analysis of variance
BDI-H, short form of the Hungarian version of the Beck Depression Inventory
CAP, cyclic alternating pattern
CTLs, control subjects
DQQ, Dream Quality Questionnaire
EEG, electroencephalography
GSQS, Groningen Sleep Quality Scale
MANCOVA, multivariate analysis of covariance
MANOVA, multivariate analysis of variance
NCAP, non-cyclic alternating pattern
NMs, nightmare subjects
NREM, non-rapid eye movement sleep
REM, rapid eye movement sleep
SPSS, Statistical Package for the Social Sciences
STAI, State-Trait Anxiety Inventory
STAI-T, State-Trait Anxiety Inventory-Trait component
SWS, slow-wave sleep
VDAS-H, the Hungarian version of the Van Dream Anxiety Scale
Acknowledgements
Work was performed at the Institute of Behavioural Sciences, Semmelweis University, H-1089, Nagyvárad tér 4, Budapest, Hungary. The current research was supported by the 2010 Research Grant of the BIAL Foundation (55/10) and the 2009 Research Grant Award of the Joint IASD/DreamScience Foundation.
Disclosure Statement
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
References
1. American Academy of Sleep Medicine. The international classification of sleep disorders: diagnostic and coding manual. 2nd ed. Westchester, IL: American Academy of Sleep Medicine, 2005.
2. Levin R, Nielsen TA. Disturbed dreaming, posttraumatic stress disorder, and affect distress: a review and neurocognitive model. Psychol Bull 2007;133:482-528.
3. Coolidge FL, Segal DL, Coolidge CM, Spinath FM, Gottschling J. Do nightmares and generalized anxiety disorder in childhood and adolescence have a common genetic origin? Behav Genet 2010;40:349-56.
4. Lancee J, Spoormaker VI, van den Bout J. Nightmare frequency is associated with subjective sleep quality but not with psychopathology. Sleep Biol Rhythms 2010;8:187-93.
5. Spoormaker VI, Schredl M, van den Bout J. Nightmares: from anxiety symptom to sleep disorder. Sleep Med Rev 2006;10:19-31.
6. Wood JM, Bootzin RR. The prevalence of nightmares and their independence from anxiety. J Abnorm Psychol 1990;99:64-8.
7. Li SX, Zhang B, Li AM, Wing YK. Prevalence and correlates of frequent nightmares: a community-based 2-phase study. Sleep 2010;33:774-80.
8. Li SX, Yu MWM, Lam SP, et al. Frequent nightmares in children: familial aggregation and associations with parent-reported behavioral and mood problems. Sleep 2011;34:487-93. 9. Schredl M. Effects of state and trait factors on nightmare frequency. Eur Arch Psychiatry Clin Neurosci 2003;253:241-7. 10. Schredl M. Nightmare frequency in patients with primary insomnia. Int J Dream Res 2009;2:85-8. 11. Schredl M. Dreams in patients with sleep disorders. Sleep Med Rev 2009;13:215-21. 12. Krakow B. Nightmare complaints in treatment-seeking patients in clinical sleep medicine settings: diagnostic and treatment implications. Sleep 2006;29:1313-9.
13. Fisher C, Byrne J, Edwards A, Kahn E. A psychophysiological study of nightmares. J Am Psychoanal Assoc 1970;18:747-82.
14. Newell SA, Padamadan H, Drake ME Jr. Neurophysiologic studies in nightmare sufferers. Clin Electroencephalogr 1992;23:203-6.
15. Germain A, Nielsen TA. Sleep pathophysiology in posttraumatic stress disorder and idiopathic nightmare sufferers. Biol Psychiatry 2003;54:1092-8.
16. Nielsen T, Paquette T, Solomonova E, Lara-Carrasco J, Colombo R, Lanfranchi P. Changes in cardiac variability after REM sleep deprivation in recurrent nightmares. Sleep 2010;33:113-22.
17. Simor P, Horváth K, Gombos F, Takács K, Bódizs R. Disturbed dreaming and sleep quality: altered sleep architecture in subjects with frequent nightmares. Eur Arch Psychiatry Clin Neurosci 2012;262:687-96.
18. Parrino L, Ferri R, Bruni O, Terzano MG. Cyclic alternating pattern (CAP): the marker of sleep instability. Sleep Med Rev 2012;16:27-45.
19. Terzano MG, Mancia D, Salati MR, Costani G, Decembrino A, Parrino L. The cyclic alternating pattern as a physiologic component of normal NREM sleep. Sleep 1985;8:137-45.
20. Bruni O, Novelli L, Miano S, Parrino L, Terzano MG, Ferri R. Cyclic alternating pattern: A window into pediatric sleep. Sleep Med 2010;11:628-36.
21. Bruni O, Ferri R, Novelli L, Finotti E, Miano S, Guilleminault C. NREM sleep instability in children with sleep terrors: the role of slow wave activity interruptions. Clin Neurophysiol 2008;119:985-92.
22. Manni R, Terzaghi M, Sartori I, Veggiotti P, Parrino L. Rhythmic movement disorder and cyclic alternating pattern during sleep: a video-polysomnographic study in a 9-year-old boy. Mov Disord 2004;19:1186-90.
23. Kutlu A, Işeri P, Selekler M, Benbir G, Karadeniz D. Cyclic alternating pattern analysis in REM sleep behavior disorder. Sleep Breath 2012; http://dx.doi.org/10.1007/s11325-012-0675-5.
24. Terzano MG, Smerieri A, Del Felice A, Giglia F, Palomba V, Parrino L. Cyclic alternating pattern (CAP) alterations in narcolepsy. Sleep Med 2006;7:619-26.
25. Bódizs R, Simor P, Csóka S, Bérdi M, Kopp MS. Dreaming and health promotion: A theoretical proposal and some epidemiological establishments. Eur J Ment Health 2008;3:35-62.
26. Simor P, Kovács I, Vargha A, Csóka S, Mangel B, Bódizs R. [Nightmares, dream anxiety and psychopathology: the validation of the Hungarian version of the Van Anxiety Scale]. Psychiatr Hung 2009;24:428-38.
27. Spielberger C, Gorsuch R, Lushene R. Manual for the state-trait anxiety inventory. Palo Alto, CA: Consulting Psychologists Press, 1970.
28. Sipos K, Sipos M, Spielberger C. A state-trait anxiety inventory (STAI) magyar változata. In: Mérei F, Szakács F, eds. Pszichodiagnosztikai Vademecum I/2. Budapest: Nemzeti Tankönyvkiadó, 1994:123-48.
29. Rózsa S, Szádoczky E, Füredi J. A Beck Depresszió Kérdőív rövidített változatának jellemzői a hazai mintán. Psychiatr Hung 16:379-97.
30. Simor P, Köteles F, Bódizs R, Bárdos G. A szubjektív alvásminőség kérdőíves vizsgálata: a Groningen Alvásminőség Skála hazai validálása. Mentálhigiéné es Pszichoszomatika 2009;10:249-61.
31. Jasper H. Report of the committee on methods of clinical examination in electroencephalography. Electroencephalogr Clin Neurophysiol 1958;10:370-5.
32. Rechtschaffen A, Kales A. A manual of standardized terminology, technique and scoring system for sleep stages of human sleep. Los Angeles: Brain Information Service. Brain Information Institute, UCLA, 1968.
33. Terzano MG, Parrino L, Smerieri A, Chervin R, Chokroverty S, Guilleminault C, et al. Atlas, rules, and recording techniques for the scoring of cyclic alternating pattern (CAP) in human sleep. Sleep Med 2002;3:187-99.
34. Iber C, Ancoli-Israel S, Chesson A, Quan SF. The AASM Manual for the scoring of sleep and associated events: rules, terminology and technical specifications. Westchester, IL: American Academy of Sleep Medicine, 2007.
35. Ferri R, Rundo F, Bruni O, Terzano MG, Stam CJ. Regional scalp EEG slow-wave synchronization during sleep cyclic alternating pattern A1 subtypes. Neurosci Lett 2006;404:352-7.
36. Ferri R, Rundo F, Bruni O, Terzano MG, Stam CJ. Dynamics of the EEG slow-wave synchronization during sleep. Clin Neurophysiol 2005;116:2783-95.
37. Ferri R, Bruni O, Miano S, Terzano MG. Topographic mapping of the spectral components of the cyclic alternating pattern (CAP). Sleep Med 2005;6:29-36.
38. Terzano MG, Parrino L. Origin and significance of the cyclic alternating pattern (CAP). Sleep Med Rev 2000;4:101-23.
39. Parrino L, Milioli G, De Paolis F, Grassi A, Terzano MG. Paradoxical insomnia: the role of CAP and arousals in sleep misperception. Sleep Med 2009;10:1139-45.
40. Schredl M, Schäfer G, Weber B, Heuser I. Dreaming and insomnia: dream recall and dream content of patients with insomnia. J Sleep Res 1998;7:191-8.
41. Aricò D, Drago V, Foster PS, Heilman KM, Williamson J, Ferri R. Effects of NREM sleep instability on cognitive processing. Sleep Med 2010;11:791-8.
42. Simor P, Pajkossy P, Horváth K, Bódizs R. Impaired executive functions in subjects with frequent nightmares as reflected by performance in different neuropsychological tasks. Brain Cogn 2012;78:274-83.
43. Ferri R, Huber R, Aricò D, et al. The slow-wave components of the cyclic alternating pattern (CAP) have a role in sleep-related learning processes. Neurosci Lett 2008;432:228-31.
44. Benca RM, Obermeyer WH, Thisted RA, Gillin JC. Sleep and psychiatric disorders: a meta-analysis. Arch Gen Psychiatry 1992;49:651-668.
45. Riemann D, Berger M, Voderholzer U. Sleep and depression–results from psychobiological studies: an overview. Biol Psychology 2001;57:67-103.
46. Mander BA, Reid KJ, Baron KG, et al. EEG measures index neural and cognitive recovery from sleep deprivation. J Neurosci 2010;30:2686-93.
47. Thomas M, Sing H, Belenky G, et al. Neural basis of alertness and cognitive performance impairments during sleepiness I: effects of 24 h of sleep deprivation on waking human regional brain activity. J Sleep Res 2000;9:335-52.
48. Wu JC, Gillin JC, Buchsbaum MS, et al. Frontal lobe metabolic decreases with sleep deprivation not totally reversed by recovery sleep. Neuropsychopharmacol 2006;31:2783-92.
49. Depue BE, Curran T, Banich MT. Prefrontal regions orchestrate suppression of emotional memories via a two-phase process. Science 2007;317:215-9.
50. Dillon DG, Pizzagalli DA. Inhibition of action, thought, and emotion: a selective neurobiological review. Appl Prev Psychol 2007;12:99-114.
51. Dolcos F, Kragel P, Wang L, McCarthy G. Role of the inferior frontal cortex in coping with distracting emotions. Neuroreport 2006;17:1591-4.