Eur Arch Psychiatry Clin Neurosci Volume 262, Issue 8, pp 687–696
DOI: 10.1007/s00406-012-0318-7
Péter Simor, Klára Horváth, Ferenc Gombos, Krisztina P. Takács, Róbert Bódizs
Péter Simor and Klára Horváth contributed equally to the article
P. Simor
Department of Cognitive Sciences, Budapest University of Technology and Economics, Egry József u. 1. Tépület./V.em., 1111 Budapest, Hungary e-mail: psimor@cogsci.bme.hu; petersimor@gmail.com
K. Horváth
Department of Experimental Psychology, University of Oxford, South Parks Road, OX1 3UD Oxford, UK
Gombos & R. Bódizs
HAS-BME Cognitive Science Research Group, Hungarian Academy of Sciences, Egry József u.1. T.ép/V.I, 1111 Budapest, Hungary
P. Takács
Institute of Psychology, University of Szeged, Egyetem utca 2, 6722 Szeged, Hungary
R. Bódizs
Institute of Behavioural Sciences, Semmelweis University, Nagyvárad tér 4, 1089 Budapest, Hungary
Abstract
Nightmares are intense, emotionally negative mental experiences that usually occur during late-night sleep and result in abrupt awakenings. Questionnaire-based studies have shown that nightmares are related to impaired sleep quality; however, the polysomnographic profile of nightmare subjects has been only scarcely investigated. We investigated the sleep architecture of 17 individuals with frequent nightmares and 23 control subjects based on polysomnographic recordings of a second night spent in the laboratory after an adaptation night. Nightmare subjects in comparison with control subjects were characterized by impaired sleep architecture, as reflected by reduced sleep efficiency, increased wakefulness, a reduced amount of slow wave sleep, and increased nocturnal awakenings, especially from Stage 2 sleep. While these differences were independent of the effects of waking psychopathology, nightmare subjects also exhibited longer durations of REM sleep that was mediated by heightened negative affect. Our results support that nightmares are related to altered sleep architecture, showing impaired sleep continuity and emotion-related increase in REM propensity.
Keywords: Nightmares, Sleep, Dreaming, EEG , Polysomnography, Sleep quality
Introduction
Nightmares are vivid, intense, and emotionally negative dream experiences that provoke abrupt awakenings especially, but not exclusively, from rapid eye movement (REM) sleep [1, 2]. Nightmares affect 2–4 % of the population on a weekly basis [2–4]; however, this rate is even higher if the awakening criterion is excluded from the definition of nightmares [5]. Indeed, research suggests that the inclusion of bad dreams without awakenings in the spectrum of dream disturbances provides a more accurate explanation regarding the relationship between dysphoric dreaming and waking negative affect [6]. Others proposed that disturbed dreaming forms a continuum from subclinical dysphoric dreaming through idiopathic nightmares to the most intense post-traumatic nightmares, where the pressure for awakening varies as a function of situational and dispositional factors as well [7, 8].
Even though nightmares often show high comorbidity with different mental disorders and clinical symptoms [7, 9–14], the direct relationship between nightmare frequency and waking psychopathology is far from being uncontroversial [15, 16]. The association between nightmare frequency and mental symptoms seems to be mediated by nightmare distress, the affective and cognitive impact of nightmares on waking functioning [6, 17]. Nevertheless, Lancee et al. [18], investigating a population with frequent nightmares, found that the mediating role of nightmare distress for the link between nightmares and waking psychopathology is only applicable to populations with high comorbidity. Moreover, their results indicate that nightmares are independent from other mental complaints, disproving the common view of nightmares as a secondary symptom of an underlying anxiety disorder. The independence of nightmares from anxiety symptoms was supported by other studies as well. Clinical observations on posttraumatic stress disorder (PTSD) suggest that symptoms of disturbed sleep (including nightmares) may persist even after the remission of waking symptoms [19]. Furthermore, a prospective study of nightmare frequency (based on dream logs) found that the prevalence of nightmares was not related to daily variations of anxiety [20]; and finally, nightmare frequency was shown to be a stable disposition with high genetic heritability, which was independent of the genetic influences of general waking anxiety [21]. Several questionnaire-based studies demonstrated that nightmares are related to poor sleep quality both in adults [5, 18, 22] and children [23]. Nightmares may deteriorate sleep quality by frequent nocturnal awakenings as well as by the fear of falling asleep or difficulties of returning to sleep [24]. According to a study based on self-reports of sleep-disordered patients, 25 % reported frequent nightmare complaints. In addition, 63 % of the nightmare sufferers indicated that their nightmares were related to disrupted sleep. Furthermore, these patients exhibited significantly worse values on sleep and health-related indexes [25]. Examining a non-clinical sample, Schredl [22] showed that while the relationship between nightmares and poor sleep quality was partly explained by trait-like effects of neuroticism and state-like effects of current stress, nightmare frequency still remained as an independent factor contributing to complaints of insomnia. Similarly, nightmares and negatively toned dreams are prevalent in patients with insomnia [26, 27], and nightmare frequency is related to the severity of insomnia symptoms [28].
Retrospective and self-report measures are an important first step in investigating the relationship between disturbed dreaming and sleep quality; however, these methods are prone to subjective biases, and cannot provide a detailed picture about sleep architecture. Nevertheless, studies examining the relationship between nightmares and sleep quality with more objective methods are scarce. An early study found that nightmare sufferers (without comorbid PTSD) were characterized by decreased total sleep time, increased amount of nocturnal awakenings, decreased slow wave sleep (SWS), and increased REM density [29].
Interestingly, a more recent polysomnographic study could not reproduce these findings, but reported higher number of periodic limb movements in PTSD and in idiopathic nightmare sufferers in comparison with controls [30]. Nonetheless, this study had relatively low sample sizes and subjects were recruited through media advertisements. These factors may limit statistical power and the representative value of the sample, respectively. Two recent studies [31, 32] investigated the effects of a partial REM deprivation procedure in a group of nightmare sufferers and healthy controls. Subjects slept 3 days in the sleep laboratory: the first night was an adaptation night, whereas on the second night subjects were woken up from the beginning of every REM sleep episode after the second (REM episode). The third night served as a REM recovery night. Nightmare sufferers exhibited decreased REM pressure, and a trend of enhanced sympathetic activation measured by heart rate variability during the first, and markedly, during the third night. According to these studies, nightmare sufferers were not characterized by impaired sleep quality. While these investigations offer valuable data about the reactive, rebound effects of REM sleep deprivation in nightmare sufferers, they could not report the macrostructure of a standard night of sleep that would be reflected by the second night spent in the laboratory without experimental manipulations.
In light of the reported associations between subjective sleep quality and nightmare frequency, and given the scarcity of polysomnographic investigations of this population, the aim of our study was to examine sleep architecture in subjects with frequent nightmares. We focused on the macrostructural characteristics of the second night spent after an adaptation night in the laboratory. We considered that sleep indexes of the second night would provide more valid picture about sleep quality compared to the first night that might be influenced by the novelty of the experimental situation, a phenomenon known as the firstnight effect [33]. Moreover, in order to investigate the primary relationship between disturbed dreaming and sleep architecture, we controlled for the possible confounding effects of waking psychopathological symptoms.
We hypothesized that:
- Nightmare subjects (NMs) will rate their sleep more fragmented in comparison with the control subjects (CTLs).
- NMs will be characterized by fragmented sleep as reflected by decreased sleep efficiency, increased duration of wakefulness after sleep onset, and decreased slow wave sleep.
- NMs in contrast to CTLs will be characterized by higher rate of nocturnal awakenings, especially from REM sleep.
- Sleep fragmentation in NMs will be independent from the effects of waking anxious and depressive symptoms.
Methods
Participants
Participants (all native Hungarians) were selected from a large pool of undergraduate students from the Budapest University of Technology and Economics and the Semmelweis University. First, they completed an online questionnaire assessing dream quality and a variety of personality factors. Subjects were told that the aim of the study was to investigate the relationship between sleep, dreams, and personality. Findings on the relationship between dream quality and personality have already been [34, 35] and will be reported elsewhere. Dreaming-related questionnaires included the Dream Quality Questionnaire (DQQ) [34], the Hungarian version of the Van Dream Anxiety Scale (VDAS-H) [36], and a 7-point Likert scale with two items; one assessing the frequency of nightmares with awakenings, and the other assessing the frequency of bad dreams without awakenings (0—almost never; 1— once or twice a year; 2—every 2–3 month; 3—once in a month; 4—twice a month; 5—once a week; 6—more than once a week). NMs were selected on the basis of the International Classification of Sleep Disorders, 2nd edition criteria [37] and Levin and Nielsen’s [7] model of disturbed dreaming, including disturbed dreamers without abrupt awakenings. Subjects reporting one or more nightmares and/or bad dreams per week in the retrospective questionnaires were assigned to the NMs group, while individuals having less than two nightmares and bad dreams during the last year were assigned to CTLs. Subjects were thoroughly interviewed about the frequency and content of their negative dream experiences. Those subjects who reported the onset of negative dream experiences in relation to a traumatic event or indicated that the content of their dreams were somehow related to a prior trauma (such as physical attack, accident, sudden death of a close relative, etc.) were excluded from the study. Finally, 17 (7 females and 10 males) NMs (Mage = 20.65 ± 1.73) and 23 (12 females and 11 males) CTLs (Mage = 21.35 ± 1.61) were included for polysomnography. (The difference in age was not significant between the two groups: U(38) = 134; Z = -1.719; p = .086). None of the subjects reported prior neurological, psychiatric, or sleep disorders or prior history of any chronic disease. NMs scored higher on the Negative Dream Affect Scale of the DQQ (NM: 7.8 ± 1.81 vs. CTL: 4.04 ± 1.69; t(38) = -6.83; p \ .001) and on the VDAS-H (NM: 19.53 ± 7.21 vs. CTL: .26 ± .62; t(16.17) = -10.99; p \ .001; equal variances not assumed), indicating at least moderately severe dream disturbances [34, 36].
The study protocol was approved by the Ethical Committee of the Semmelweis University. The subjects received monetary compensation (approximately 20 Euros in Hungarian Forints) for their participation in the sleep laboratory investigations. Written informed consent was obtained.
Psychometric tests
The STAI [38] is a widely used self-report instrument that differentiates between the temporary condition of state anxiety and the longstanding quality of trait anxiety. We used the 20-item Hungarian version of the STAI trait anxiety questionnaire (STAI-T) to assess general levels of anxiety [39]. The questions are scored on a 4-point Likert scale. The scale has proven to be valid and reliable tool for the measurement of trait anxiety, showing excellent internal consistency in different studies [40].
We used the short form of the Hungarian version of the Beck Depression Inventory (BDI-H) [41] in order to measure the extent of waking depressive symptoms in our subjects. The 9-item BDI-H is a one-dimensional scale assessing different symptoms of depression including social withdrawal, indecision, sleep disturbance, fatigue, intense worry about bodily symptoms, work inhibition, pessimism, lack of satisfaction, self-accusation. The items are scored on a 4-point Likert scale. The instrument showed good internal consistency, and high specificity and sensitivity for screening depression [41].
The Hungarian version of the Groningen Sleep Quality Scale (GSQS) was used for measuring subjective sleep quality [42]. The 14-item questionnaire measures the extent of subjective sleep fragmentation by a binary scale. The internal reliability and validity measures of the scale indicated that the questionnaire was an adequate tool for assessing subjective sleep quality [42].
Procedure
Polysomnographic recordings were performed in the sleep research laboratory of the Semmelweis University for 2 consecutive nights. (The first night served as the adaptation night). Subjects were not allowed to drink alcohol and take drugs (except contraceptives) on the day and the previous day of the examination. They were asked to avoid napping and consuming caffeine on the afternoon of the sleep recordings. Subjects completed the short version of the Beck Depression Inventory upon arrival. (The STAI-T was completed previously during the selection procedure.) The timing of lights off was between 11.00 PM and 1.00 AM depending on each participant’s preferred bedtime. Morning awakenings were scheduled after 9 h of undisturbed sleep except if participants woke up earlier spontaneously. In the morning, participants were asked to complete the Groningen Sleep Quality Scale and to report their dreams. One of the NMs reported a nightmare and two of them had bad dreams in the laboratory.
Polysomnography
During the standard polysomnographic examination on both nights, subjects were fitted with 19 EEG (Fp1, Fp2, F3, F4, Fz, F7, F8, C3, C4, Cz, P3, P4, Pz, T3, T4, T5, T6, O1, O2) electrodes according to the 10–20 electrode placement system [43] as well as with 2 EOG electrodes (bipolar channel) monitoring vertical and horizontal eye movements, 2–2 EMG electrodes (bipolar channels) for chin and for the anterior tibialis muscles, 2 ECG electrodes according to standard lead I, in addition to the thoracic and abdominal respiration sensors. Gold-coated Ag/AgCl EEG cup electrodes were fixed with EC2 Grass Electrode Cream (Grass Technologies, USA) and referred to the mathematically linked mastoids. Impedances were kept below 8 kX. Signals were collected, prefiltered (.33–1,500 Hz, 40 dB/decade anti-aliasing hardware input filter), amplified, and digitized with 4,096 Hz/channel sampling rate (synchronous) with 12 bit resolution by using the 32 channel EEG/polysystem (Brain-Quick BQ 132S, Micromed, Italy). A further 40-dB/decade anti-aliasing digital filter was applied by digital signal processing which lowpass filtered the data at 450 Hz. After this, the digitized and filtered EEG was subsequently undersampled at 1,024 Hz. Wakefulness and sleep stages of the second night were identified manually according to the criteria of Rechtschaffen and Kales [44] by two experienced sleep researchers who were blind to the group membership of the subjects. A program developed by our laboratory was used to output the following sleep architecture variables: Wake time after sleep onset (WASO), Sleep efficiency (Sleep time/Time in bed), Sleep latency (period between lights off and the first epoch scored as Stage 2 sleep), absolute and relative (to the sleep time) duration of Non-REM (NREM) sleep, Stage 1, Stage 2, SWS (including Stage 3 and 4), REM sleep, and REM latency (period between sleep onset and the first epoch scored as REM sleep). We computed the measure of REM density (the frequency of eye movements (EM) during the REM phase) which was quantified as the total number of EM in REM/duration of REM sleep (in seconds). The scoring of eye movements was based on visual detection of the EOG by a trained researcher who was blind to the group membership of the subjects. Rapid left and right EMs and saccades in the same direction of gaze with the minimum amplitude of 50(lV) were counted as separate eye movements. The number of awakenings from different stages (Stage 2, SWS, REM) was also computed.
Statistical analyses
All statistical procedures were carried out with the Statistical Package for the Social Sciences (SPSS) version 19 (IBM). Mean scores of the psychometric tests were compared with independent samples t test, and, if the criterion for homogeneity of variance was violated, we applied the Welch test. For those psychometric variables that were not normally distributed (according to the Shapiro–Wilk test), the Mann–Whitney U test was used. Several sleep variables (Sleep efficiency, WASO, Sleep latency, Relative Stage 1 duration, REM latency, number of awakenings) were characterized by positively skewed distributions. These variables were transformed to a natural logarithmic scale in order to normalize their distribution. Sleep variables as dependent factors were entered to multivariate analysis of variance (MANOVA) with group as an independent factor. To control the possible effects of the BDI-H and STAI-T on the group differences in sleep variables, multivariate analysis of covariance (MANCOVA) was conducted with the BDI-H and STAI-T scores as covariates. Since the EOG recordings of 3 subjects (2 CTL and 1 NM) were too noisy hindering the detection of EMs for the whole sample, the group difference in REM density was analyzed with univariate ANOVA without and with STAI-T and BDI-H as covariates. To compare the number of awakenings across groups, t-tests and univariate analysis of covariance (ANCOVA) were carried out. Pearson correlation coefficients were computed in order to analyze the relationship between waking depressive and anxiety symptoms and the absolute duration of sleep stages.
Results
Psychometric measures
Besides the group differences regarding the VDAS-H and the DQQ Negative Dream Affect scale (see ‘‘Participants’’ section), NMs reported increased levels of depressive symptoms and waking anxiety as evidenced by higher scores on the BDI-H (NM: 15.59 ± 3.83 vs. CTL: 10.87 ± 1.63; t(21.39) = -4.321; p \ .001; equal variances not assumed) and STAI-T questionnaires (NM: 49.94 ± 7.66 vs. CTL: 33.3 ± 8.14; t(38) = -5.971; p \ .001). These scores reflect mild, sub-clinical depression [41] and moderate levels of trait anxiety [39] in NMs. No significant difference was found between the two groups regarding subjective sleep fragmentation measured by the Groningen Sleep Quality Scale (NM: 3.76 ± 3.05 vs. CTL: 2.78 ± 2.17; U(38) = 158; Z = -1.041; p = .298).
Sleep architecture
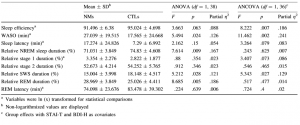
In order to normalize their distribution, some of the sleep variables (see Table 1) were logarithmized. The MANOVA revealed that NMs differed significantly from CTLs in the sleep variables (F(9, 30) = 2.25; p = .046). According to the univariate tests, NMs had significantly longer WASO and their sleep tended to be less efficient. Moreover, their relative NREM and SWS duration were significantly shortened, whereas their relative REM duration was significantly longer in comparison with the CTLs. Sleep latency, relative duration of Stage 1 and Stage 2, and REM latency did not differ between the two groups. Means of the groups, p values, and effect sizes are shown in Table 1. In order to control for the confounding effects of anxiety and depression scores, we carried out MANCOVA with STAI-T and BDI-H scores as covariates in the model. NMs still differed significantly from CTLs (F(9, 28) = 2.311; p = .043). Significant group differences emerged for WASO, sleep efficiency, and SWS duration. NMs spent more time awake after falling asleep, their sleep was less efficient, and they spent less time in SWS in comparison with the CTLs. Moreover, these group differences were independent of the effects of questionnaire measures of depressive and anxiety symptoms. In contrast, by the inclusion of the two covariates the difference in relative NREM and REM duration did not remain significant, whereas sleep latency and the relative duration of Stage 1 sleep showed a marginal association with group. NMs had longer Stage 1 sleep, as well as they fell asleep more slowly. While the decreased relative NREM duration and the increased relative REM duration in NMs seem to be mediated by waking anxiety and depressive symptoms, longer duration of Stage 1 sleep and sleep latency were not a function of depressive symptoms and/or waking anxiety. Effect sizes and p values can be observed in Table 1. Furthermore, we conducted univariate ANOVA to analyze the group differences regarding REM density without and with the control for STAI-T and BDI-H scores. Significant differences were found neither without (F(1, 38) = .006; p = .937) nor with the control for the psychometric variables (F(1, 35) = .193; p = .663).
Nocturnal awakenings
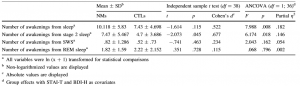
In addition, to clarify whether an increased number of awakenings are responsible for the altered duration of different sleep stages, the numbers of awakenings were also compared between the groups. Because these variables were not normally distributed, ln (x ? 1) transformation was applied to normalize their distribution. Although the transformed variables still deviated from normality, the kurtoses of the distributions, which could have great effect on the robustness of the F test, were in acceptable range (-.6 to .4). Group comparisons revealed that NMs woke up significantly more, particularly from Stage 2 sleep. Group differences were more pronounced after controlling for waking the STAI-T and BDI scores. The number of awakenings from REM and SWS did not differ significantly between the two groups. Detailed results are shown in Table 2.
Table 1 Differences in sleep architecture between NMs and CTLs Mean ± SDb
Table 2 Differences in the number of awakenings between NMs and CTLs
Associations between the absolute duration of sleep stages and questionnaire measures of waking depression/anxiety
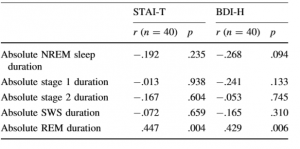
Due to the difference between the results of the ANOVA and the ANCOVA, we analyzed further the associations of the lengths of different sleep stages with the STAI-T and BDI-H scores by computing Pearson correlation coefficients between the variables. Correlations were computed across the two groups. In order to examine these associations, we used the absolute length of the sleep stages. While in the previous analysis we used the relative amount of different sleep stages, here we decided to analyze the absolute amounts (length) because these values are independent of the duration of sleep, and therefore, from each other. Only absolute REM sleep duration showed significant correlations with STAI-T and BDI-H scores. None of the other variables correlated significantly with the psychometric scales. p and r values are shown in Table 3.
Discussion
We compared the sleep architecture of young subjects with frequent nightmares and healthy controls as reflected by the second night spent in the sleep laboratory. Sleep architecture clearly differentiated the two groups. NMs exhibited worse sleep quality than CTLs, showing reduced sleep efficiency, increased wakefulness after falling asleep, and reduced percentage of SWS. Moreover, NMs showed a trend regarding longer sleep latency and increased proportion of Stage 1 sleep. Furthermore, NMs were characterized by an increased number of nocturnal awakenings in Stage 2 sleep. Even though reduced SWS was characteristic in alcohol dependence and major depression [45], these differences between the two groups were independent of the confounding effects of sub-clinical waking psychopathology, suggesting that the relationship between
Table 3 Pearson correlation coefficients between psychometric tests and absolute duration of sleep stages across groups STAI-T
nightmares and impaired sleep continuity is not a function of waking depressive symptoms or anxiety.
In contrast to our first hypothesis, NMs in comparison with CTLs did not rate their sleep as more fragmented according to the self-report measure of subjective sleep quality. While several studies applying retrospective questionnaires reported impaired subjective sleep quality in relation to nightmares [5, 18, 22, 24, 25], to the best of our knowledge, subjective sleep quality of NMs was not previously assessed in laboratory conditions. The incidence of disturbed dreaming is relatively low under laboratory and clinical settings even in traumatized subjects with severe post-traumatic nightmares [2, 46], and it is possible that the laboratory settings may influence—in this case, beneficially—the subjective quality of sleep as well. By all means, this finding indicates that our subjects were adequately adjusted to the artificial settings of the sleep laboratory and for the second night they had overcome the somewhat disturbing effects of polysomnography.
Interestingly, while NMs reported adequate subjective sleep quality, objective sleep quality was impaired in this group. In consistence with our second and third hypothesis, we found reduced sleep efficiency, increased WASO, decreased SWS, and more nocturnal awakenings in NMs in comparison with CTLs. Moreover, a trend for longer sleep latency and increased Stage 1 sleep also reflects impairments of sleep regulation in NMs. We should note that while NMs showed consistent differences in these measures of sleep continuity, the alterations were only slightly below the normal range for this age group [47].
NMs also exhibited slightly longer durations of relative REM sleep and consequently slightly shortened relative NREM sleep in contrast to CTLs; although after controlling for depression and anxiety, the differences between the groups were not significant. Shortened REM latency, increased REM duration, and increased REM density are considered to be stabile biological markers of affective dysregulation in depression and other mood disorders [48–50]. However, we did not find increased REM density in NMs which may indicate the dissimilarity of frequent nightmares from depression. Furthermore, brain imaging as well as behavioral research indicates that REM sleep is intimately related to the activation of emotion-processing networks [51, 52]. Frequent nightmares are often comorbid with disorders of emotional regulation [7], and nightmares are experiences involving intense negative emotions. Therefore, it is plausible that in NMs the relative increase in REM pressure in the second half of the night was mediated by the heightened negative affect. Accordingly, we found that depressive symptoms and trait anxiety as measured by the BDI-H and the STAI-T, respectively, showed medium-size correlations with the length of REM sleep.
In sum, we found impaired sleep continuity with reduced SWS and increased REM pressure in NMs, but while alterations in sleep continuity were independent associates with disturbed dreaming, heightened REM activity seemed to be a function of comorbid affective dysregulation. Our results cohere with earlier reports of Fisher and colleagues [29], but are inconsistent to the results of a more recent study by Germain and Nielsen [30], which did not find altered sleep architecture in idiopathic nightmare sufferers. Nevertheless, in this study, three groups (post-traumatic nightmare sufferers, idiopathic nightmare sufferers, and healthy controls) were compared with relatively low sample sizes limiting statistical power for testing group differences. Moreover, their nightmare subjects were elder in comparison with the subjects of the present study and were recruited by media advertisements instead of the questionnaire-based selection process that we applied. And finally, the authors did not control for the confounding effects of waking psychopathology that might have influenced sleep parameters.
Spoormaker [2] and Lancee et al. [18] proposed that nightmares should be viewed as a sleep disorder instead of a secondary symptom of an underlying mental complaint. This assumption may be supported by genetic twin studies carried out on NMs [21, 53]. Our results demonstrating that impaired sleep continuity in subjects with frequent nightmares is independent of the effects of waking depressive symptoms or trait anxiety clearly support this claim. Indeed, our analyses showed that the statistical control for the effects of waking psychopathological symptoms resulted in a better model explaining the group differences of altered sleep architecture regarding NREM sleep. Nevertheless, waking symptoms of psychopathology have a profound impact on REM sleep in NMs due to the higher levels of depression and anxiety scores that characterize them. Thus, it could be possible that nightmare disorder is the consequence of the combination of two at least partly independent factors, the impaired NREM continuity and the increased REM pressure stemmed from the increase of depressive and anxious symptoms that NMs experience.
Instead of the prevailing view that nightmare disorder affects mainly REM sleep [31, 37], our results indicate that NMs have impaired sleep regulation during NREM sleep. Longer sleep latency, increased number of nocturnal awakenings, and reduced SWS reflect a more aroused, more alert brain state resulting in less restorative and less efficient sleep. Since the majority of awakenings occurred in the second Stage of NREM sleep, it is highly probable that the reduced amount of SWS in NMs is due to these relatively short nocturnal awakenings that may hinder the appearance of slower neural oscillations during NREM sleep and prevent sleep to reach deeper stages. It is well known that SWS appears predominantly in the first third of the night [47]. Later as sleep progresses, the neural apparatus may not recover the deficit of slow activity because of the heightened pressure of REM sleep related to comorbid affective dysregulation.
Disrupted sleep in NMs may resemble sleep patterns found in chronic insomnia stemmed from hyperarousal processes during sleep [54, 55]. The possible overlap between nightmares and insomnia is supported by an epidemiologic study that showed higher prevalence (18.3 %) of nightmares in subjects complaining about insomnia symptoms [26] compared to the population (2–4 %). Moreover, one study [30] reported similar patterns of elevated periodic limb movements in PTSD and idiopathic nightmare disorder indicating sleep-related hyperarousal in PTSD and idiopathic nightmares sufferers [30]. Therefore, it is feasible that impaired sleep continuity in our NMs originates from heightened arousal; however, future studies with more refined quantitative analysis on the neural oscillations as well as the arousal processes during sleep should examine the overlapping patterns or dissimilarities between these pathological states.
Our results indicate that NMs are not aware of their impaired sleep continuity. This is an interesting finding since the opposite is reported in samples of subjective insomnia patients, who tend to overestimate the amount of wake time disrupting sleep [55]. Sleep state misperception in insomniacs is related to the dissociation between sleepinducing and arousing mechanisms—with abnormally increased cortical arousal—and consequently to enhanced levels of sensory and cognitive processing during sleep [55, 56]. While it is possible that the ‘‘harmony’’ of sleeppromoting and arousing mechanisms is also impaired in NMs, we speculate that in their case, cortical hyperarousal is not resulting in increased sensory processing of external information. In contrast, NMs may engage cortical resources toward internally generated sensorial, emotional, and cognitive processes that constitute the raw material for intense dream experiences [30, 57].
Nightmares are thought to impair sleep quality by frequent nocturnal awakenings as well as by fear of falling or returning to sleep [24]. However, an inverse mechanism could be also plausible. Fragmented sleep, with frequent arousals and awakenings, reflecting impaired sleep continuity, may also trigger the appearance of dysphoric dreams, especially in case of comorbid trait-like effects of affective dysregulation or state-like effects of current stress. In addition, the high number of awakenings and the more superficial sleep may promote the recall of dreams including dysphoric ones. Furthermore, impaired sleep quality may also have deleterious effects on affective processes during waking. For instance, a recent longitudinal study with adolescents showed that poor sleep quality is predictive of impaired emotional information processing abilities [58]. Impaired emotion processing may compromise emotional regulation, and thus lead to heightened negative affect.
While our study does not allow for inferences on the mechanism of nightmare generation, it clearly indicates that impaired sleep continuity is an integral part of this disorder. Therefore, we consider that the interrelation between disturbed dreaming and altered sleep regulation requires further investigations. Apart from the short nocturnal awakenings, examining microarousals that do not lead to awakenings in NREM sleep would provide a more detailed picture about impaired sleep regulation in NMs. Moreover, examining the sleep stages with more refined quantitative methods would foster our understanding of the presumably altered neural oscillatory activity in this population.
Since we were interested in the ‘‘undisturbed’’ sleep architecture of individuals with frequent nightmares, we did not wake up our subjects in order to collect dream reports. Consequently, our study does not provide any information regarding sleep parameters during nightmares. Moreover, only 3 of our subjects reported dysphoric dream experiences in consistence to earlier results showing the attenuation of nightmares in laboratory settings [2]. Ambulant sleep recordings or polysomnographic measurements with more adaptation nights would be more adequate methods in order to examine sleep parameters in NMs. Additionally, we did not acquire data on circadian rhythms that might be related to altered sleep patterns in NMs. A recent study indicates that nightmares are related to eveningness chronotype in females [59]; therefore, it is possible that the predominance of this among NMs influenced our results. Nevertheless, it seems unlikely that sleep disruptions were the result of eveningness chronotype since subjects went to bed at their preferred bedtime and sleep pressure might have been increased for the second night if sleep curtailment occurred for the first night. And finally, examining a student sample may be advantageous because of the relative homogeneity of our subjects, but on the other hand, we must be careful in generalizing our results to other populations. In spite of these limitations, our study provides relevant empirical data on the relationship between nightmares and altered sleep architecture contributing to our understanding of this scarcely investigated but peculiar oneiric experience.
Acknowledgments
The present research was supported by the 2010 Research Grant of the BIAL Foundation (55/10) and the 2009 Research Grant Award of the Joint IASD/DreamScience Foundation. The authors thank Attila Keresztes for his valuable comments on the manuscript.
Conflict of interest
The authors declare no conflicts of interest.
References
- American Psychiatric Association (2000) Diagnostic and statistical manual of mental disorders: DSM-IV-TR. 4th text revision edn. American Psychiatric Publishing, Washington, DC
- Spoormaker VI, Schredl M, van den Bout J (2006) Nightmares: from anxiety symptom to sleep disorder. Sleep Med Rev 10(1): 19–31
- Bixler EO, Kales A, Soldatos CR, Kales JD, Healey S (1979) Prevalence of sleep disorders in the Los Angeles metropolitan area. Am J Psychiatry 136(10):1257–1262
- Schredl M (2010) Nightmare frequency and nightmare topics in a representative German sample. Eur Arch Psychiatry Clin Neurosci 260(8):565–570
- Li SX, Zhang B, Li AM, Wing YK (2010) Prevalence and correlates of frequent nightmares: a community-based 2-phase study. Sleep 33(6):774–780
- Blagrove M, Farmer L, Williams E (2004) The relationship of nightmare frequency and nightmare distress to well-being. J Sleep Res 13(2):129–136
- Levin R, Nielsen TA (2007) Disturbed dreaming, posttraumatic stress disorder, and affect distress: a review and neurocognitive model. Psychol Bull 133(3):482–528
- Zadra A, Pilon M, Donderi DC (2006) Variety and intensity of emotions in nightmares and bad dreams. J Nerv Ment Dis 194(4): 249–254
- Agargun MY, Besiroglu L, Cilli AS, Gulec M, Aydin A, Inci R, Selvi Y (2007) Nightmares, suicide attempts, and melancholic features in patients with unipolar major depression. J Affect Disord 98(3):267–270
- Agargun MY, Kara H, Ozer OA, Selvi Y, Kiran U, Kiran S (2003) Nightmares and dissociative experiences: the key role of childhood traumatic events. Psychiatry Clin Neurosci 57(2): 139–145
- Roberts J, Lennings CJ (2006) Personality, psychopathology and nightmares in young people. Pers Individ Dif 41(4):733–744
- Simor P, Csóka S, Bódizs R (2010) Nightmares and bad dreams in patients with borderline personality disorder: fantasy as a coping skill? Eur J Psychiat 24(1):28–37
- Krakow B, Schrader R, Tandberg D, Hollifield M, Koss MP, Yau CL, Cheng DT (2002) Nightmare frequency in sexual assault survivors with PTSD. J Anxiety Disord 16(2):175–190
- Tanskanen A, Tuomilehto J, Viinama¨ki H, Vartiainen E, Lehtonen J, Puska P (2001) Nightmares as predictors of suicide. Sleep 24(7):844–847
- Spoormaker VI, van den Bout J (2005) Depression and anxiety complaints; relations with sleep disturbances. Eur Psychiatry 20(3):243–245
- Levin R, Fireman G (2002) Nightmare prevalence, nightmare distress, and self-reported psychological disturbance. Sleep 25(2): 205–212
- Belicki K (1992) Nightmare frequency versus nightmare distress: relations to psychopathology and cognitive style. J Abnorm Psychol 101(3):592–597
- Lancee J, Spoormaker VI, Van den Bout J (2010) Nightmare frequency is associated with subjective sleep quality but not with psychopathology. Sleep Biol Rhythms 8(3):187–193
- Spoormaker VI, Montgomery P (2008) Disturbed sleep in posttraumatic stress disorder: secondary symptom or core feature? Sleep Med Rev 12(3):169–184
- Wood JM, Bootzin RR (1990) The prevalence of nightmares and their independence from anxiety. J Abnorm Psychol 99(1):64–68
- Coolidge FL, Segal DL, Coolidge CM, Spinath FM, Gottschling J (2010) Do nightmares and generalized anxiety disorder in childhood and adolescence have a common genetic origin? Behav Genet 40(3):349–356
- Schredl M (2003) Effects of state and trait factors on nightmare frequency. Eur Arch Psychiatry Clin Neurosci 253(5):241–247
- Li SX, Yu MW, Lam SP, Zhang J, Li AM, Lai KY, Wing YK (2011) Frequent nightmares in children: familial aggregation and associations with parent-reported behavioral and mood problems. Sleep 34(4):487–493
- Krakow B, Tandberg D, Scriggins L, Barey M (1995) A controlled comparison of self-rated sleep complaints in acute and chronic nightmare sufferers. J Nerv Ment Dis 183(10):623–627
- Krakow B (2006) Nightmare complaints in treatment-seeking patients in clinical sleep medicine settings: diagnostic and treatment implications. Sleep 29(10):1313–1319
- Ohayon MM, Morselli PL, Guilleminault C (1997) Prevalence of nightmares and their relationship to psychopathology and daytime functioning in insomnia subjects. Sleep 20(5):340–348
- Schredl M, Schafer G, Weber B, Heuser I (1998) Dreaming and insomnia: dream recall and dream content of patients with insomnia. J Sleep Res 7(3):191–198
- Schredl M (2009) Nightmare frequency in patients with primary insomnia. Int J Dream Res 2(2):85–88
- Fisher C, Byrne J, Edwards A, Kahn E (1970) A psychophysiological study of nightmares. J Am Psychoanal Assoc 18(4): 747–782
- Germain A, Nielsen TA (2003) Sleep pathophysiology in posttraumatic stress disorder and idiopathic nightmare sufferers. Biol Psychiatry 54(10):1092–1098
- Nielsen TA, Paquette T, Solomonova E, Lara-Carrasco J, Popova A, Levrier K (2010) REM sleep characteristics of nightmare sufferers before and after REM sleep deprivation. Sleep Med 11(2):172–179
- Nielsen T, Paquette T, Solomonova E, Lara-Carrasco J, Colombo R, Lanfranchi P (2010) Changes in cardiac variability after REM sleep deprivation in recurrent nightmares. Sleep 33(1):113–122
- Agnew HWJ, Webb WB, Williams RL (1966) The first night effect: an EEG study of sleep. Psychophysiology 2(3):263–266 34. Bódizs R, Simor P, Csóka S, Bérdi M, Kopp MS (2008) Dreaming and health promotion: a theoretical proposal and some epidemiological establishments. Eur J Ment Health 3(1):35–62
- Simor P, Köteles F, Sándor P, Petke Z, Bódizs R (2011) Mindfulness and dream quality: the inverse relationship between mindfulness and negative dream affect. Scand J Psychol 52(4): 369–375
- Simor P, Kovacs I, Vargha A, Csoka S, Mangel B, Bodizs R (2009) Nightmares, dream anxiety and psychopathology: the validation of the Hungarian version of the Van Anxiety Scale. Psychiatr Hung 24(6):428–438
- American Academy of Sleep Medicine (2005) The international classification of sleep disorders: diagnostic and coding manual, 2nd edn. American Academy of Sleep Medicine, Westchester
- Spielberger C, Gorsuch R, Lusgene R (1970) Manual for the state-trait anxiety inventory. Consulting Psychologists Press, Palo Alto
- Sipos K, Sipos M, Spielberger CD (1994) A State-Trait Anxiety Inventory (STAI) magyar változata. In: Mérei F, Szakács F (eds) Pszichodiagnosztikai Vademecum I/2. Nemzeti Tankönyvkiadó, Budapest, pp 123–148
- Köteles F, Szemerszky R, Freyler A, Bárdos G (2011) Somatosensory amplification as a possible source of subjective symptoms behind modern health worries. Scand J Psychol 52(2): 174–178
- Rózsa S, Szádoczky E, Füredi J (2001) A Beck Depresszió Kérd} óıv rövidített változatának jellemz} oi a hazai mintán. Psychiatria Hungarica 16(4):379–397
- Simor P, Köteles F, Bódizs R, Gy B (2009) A questionnaire based study of subjective sleep quality: the psychometric evaluation of the Hungarian version of the Groningen Sleep Quality Scale. Mentálhigiéné és Pszichoszomatika 10(3):249–261
- Jasper H (1958) Report of the committee on methods of clinical examination in electroencephalography. Electroenceph Clin Neurophysiol 10:370–375
- Rechtschaffen A, Kales A (1968) A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. UCLA, Brain Information Service, Los Angeles
- Brower KJ, Hoffmann R, Conroy DA, Arnedt JT, Armitage R (2011) Sleep homeostasis in alcohol-dependent, depressed and healthy control men. Eur Arch Psychiatry Clin Neurosci 261(8): 559–566
- Woodward SH, Arsenault NJ, Murray C, Bliwise DL (2000) Laboratory sleep correlates of nightmare complaint in PTSD inpatients. Biol Psychiatry 48(11):1081–1087
- Rechtschaffen A, Siegel JM (2000) Sleep and dreaming. In: Kandel ER, Schwartz JH, Jessel TM (eds) Principles of neuroscience, 4th edn. McGraw-Hill, New York
- Benca RM, Okawa M, Uchiyama M, Ozaki S, Nakajima T, Shibui K, Obermeyer WH (1997) Sleep and mood disorders. Sleep Med Rev 1(1):45–56
- Riemann D, Berger M, Voderholzer U (2001) Sleep and depression–results from psychobiological studies: an overview. Biol Psychol 57(1–3):67–103
- Buysse DJ, Tu XM, Cherry CR, Begley AE, Kowalski J, Kupfer DJ, Frank E (1999) Pretreatment REM sleep and subjective sleep quality distinguish depressed psychotherapy remitters and nonremitters. Biol Psychiatry 45(2):205–213
- Gujar N, McDonald SA, Nishida M, Walker MP (2011) A role for REM sleep in recalibrating the sensitivity of the human brain to specific emotions. Cereb Cortex 21(1):115–1123
- Maquet P, Ruby P, Maudoux A, Albouy G, Sterpenich V, DangVu T, Desseilles M, Boly M, Perrin F, Peigneux P, Laureys S (2005) Human cognition during REM sleep and the activity profile within frontal and parietal cortices: a reappraisal of functional neuroimaging data. Prog Brain Res 150:219–227
- Hublin C, Kaprio J, Partinen M, Koskenvuo M (1999) Nightmares: familial aggregation and association with psychiatric disorders in a nationwide twin cohort. Am J Med Genet 88(4): 329–336
- Krystal AD, Edinger JD, Wohlgemuth WK, Marsh GR (2002) NREM sleep EEG frequency spectral correlates of sleep complaints in primary insomnia subtypes. Sleep 25(6):630–640
- Riemann D, Spiegelhalder K, Feige B, Voderholzer U, Berger M, Perlis M, Nissen C (2010) The hyperarousal model of insomnia: a review of the concept and its evidence. Sleep Med Rev 14(1):19–31
- Feige B, Al-Shajlawi A, Nissen C, Voderholzer U, Hornyak M, Spiegelhalder K, Kloepfer C, Perlis M, Riemann D (2008) Does REM sleep contribute to subjective wake time in primary insomnia? A comparison of polysomnographic and subjective sleep in 100 patients. J Sleep Res 17(2):180–190
- Nielsen TA, Stenstrom P (2005) What are the memory sources of dreaming? Nature 437(7063):1286–1289
- Soffer-Dudek N, Sadeh A, Dahl RE, Rosenblat-Stein S (2011) Poor sleep quality predicts deficient emotion information processing over time in early adolescence. Sleep 34(11):1499–1508
- Nielsen T (2010) Nightmares associated with the eveningness chronotype. J Biol Rhythms 25(1):53–62