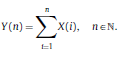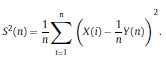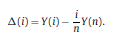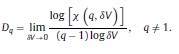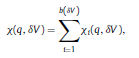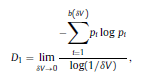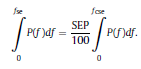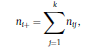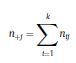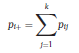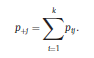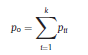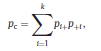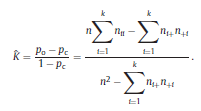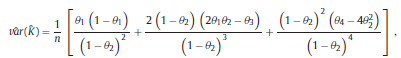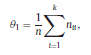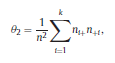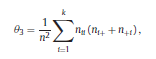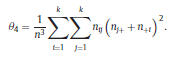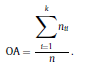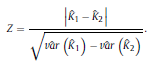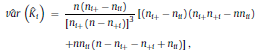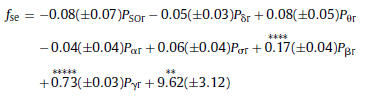|
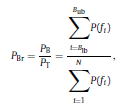
Béla Weissa, Zsófia Clemensb, Róbert Bódizsc,d, Péter Halásza aFaculty of Information Technology, Pázmány Péter Catholic University, Práter u. 50/a, 1083 Budapest, Hungary Received: 22 July 2010 Received in revised form: 30 November 2010 Accepted: 7 December 2010 ABSTRACTFractal nature of the human sleep EEG was revealed recently. In the literature there are some attempts to relate fractal features to spectral properties. However, a comprehensive assessment of the relationship between fractal and power spectral measures is still missing. Therefore, in the present study we investigated the relationship of monofractal and multifractal EEG measures (H and D) with relative band powers and spectral edge frequency across different sleep stages and topographic locations. In addition wetested sleep stage classification capability of these measures according to different channels.We found that cross-correlations between fractal and spectral measures as well as between H and D exhibit specific topographic and sleep stage-related characteristics. Best sleep stage classifications were achieved by estimating measureD in temporal EEG channels both at group and individual levels, suggesting that assessing multifractality might be an adequate approach for compact modeling of brain activities. KeywordsFractal analysis; Power spectral analysis; Topography; Cross-correlation; Sleep stage classification 1. Introduction With advent of relatively cheap and high performance personal computers sophisticated and computation demanding time series analysis methods became available to a broad brain research community. Application of chaos theory and non-linear time series methods gave a deeper insight into brain dynamics reflected by EEG signals. For a comprehensive recent review on non-linear analysis of EEG we refer to ref. [90]. This approach relies on a transition from the time domain to the phase space and generation of trajectories for EEG time series using different embedding techniques. Inferences about brain dynamics were drawn by estimating different characteristics of trajectory attractors using measures such as the correlation dimension D2, the fractal dimension Df, the largest Lyapunov exponent L1, etc. Early results were promising and suggested a deterministic nature of brain dynamics with a rather low-dimensional chaotic behavior in physiological and pathological conditions that could not be revealed using simple linear methods such as power spectral analyses. Filtered noise, however,can mimic the signatures of deterministic chaos [77]. This latter finding necessitated a revision of results obtained by non-linear techniques. Surrogate data analyses [83] did not entirely support early results on the low-dimensional chaotic behavior of the brain. This was in agreement with the finding that the relatively high complexity of the EEG signals does not allow a reliable dissociation of its waxing and waning oscillations exceeding 2–15 s from that of the filtered white noise [14,69,89]. As a consequence alternative approaches were developed including novel non-linear and stochastic time series analysis methods. 2. Materials and methods 2.1. Subjects and EEG recordings Twenty-two healthy subjects with no sleep disturbances, free of drugs and medications as assessed by an interview and questionnaires on sleeping habits and health participated in the study (age: 17–55 years, mean±S.D.: 31±9 years, 11 males and 11 females). The study was approved by the ethical committee of the Semmelweis University and subjects gave written informed consent to participation. Sleep was recorded in the sleep laboratory for two consecutive nights. The timing of lights off was determined by the subjects, and morning awakenings were spontaneous. On average subjects spent 462.39±69.01 (mean±S.D.) minutes in sleep during the second night. Sleep was recorded by standard polysomnography, including electroencephalography (Fp1, Fp2, F3, F4, Fz, F7, F8, C3, C4, Cz, P3, P4, T3, T4, T5, T6, O1 and O2 electrodes), electrooculography (EOG), bipolar submental electromyography (EMG) and electrocardiography (ECG). EEG electrodes were referenced to the contralateral mastoid. Midline EEG electrodes were referenced to the right mastoid. Impedance of the EEG electrodes was kept below 5 k. Signals were collected, pre-filtered, amplified and digitized at a sampling rate of fs = 249 Hz using the 30 channel Flat Style SLEEP La Mont Headbox with implemented second order filters at 0.5 Hz (high pass) and 70 Hz (low pass) as well as the HBX32-SLP 32 channel preamplifier (La Mont Medical Inc., USA). Additionally, a 50Hz digital notch filtering was performed by means of the DataLab acquisition software (Medcare, Iceland). 2.2. EEG processing Pre-processing and feature estimation were accomplished in a self-developed EEG visualization and processing toolbox under Matlab2009b (MathWorks, Natick, MA,USA). Statistical analyses were performed using STATISTICA (StatSoft, Inc., Tulsa, OK, USA) and Matlab2009b. 2.2.1. Pre-processing 2.2.2. Fractal analysis 2.2.2.1. Estimation of the Hurst exponent. 2.2.2.2. Estimation of multifractal spectra. 2.2.3. Power spectral measures (PSMs) where PT is the total power of the time series, PB is the total power of the B frequency band, fi = (i−1)Δf with a maximal value of fs/2 when i =N, Bub and Blb are indices corresponding to the upper and lower boundary frequencies of the B band, respectively. The analyzed bands were as follows: SO: (0.5–1] Hz, δ: (1–4] Hz, Θ: (4–8] Hz,α: (8–11] Hz, σ : (11–16] Hz, β: (16–30] Hz, γ: (30–70] Hz. These frequency bands are thought to represent specific EEG patterns. Slow oscillations (SOs) were assessed separately from delta band activity given their distinct characteristics [3,66]. In this study SEP and cut-off fcse frequency parameters were set to 95% and 70 Hz, respectively. According to Eq. (14) and the nature of sleep EEG, lower fse values can be predicted for deeper sleep stages since during these states the power spectrum is biased towards lower frequencies. 2.3. Statistical analysis 2.3.1. Topographic distribution of EEG measures across different sleep stages 2.3.2. Cross-correlation analysis 2.3.3. Hierarchical clustering 2.3.4. Linear discriminant analysis 2.3.4.1. Confusion matrix. while is the total number of segments classified to the jth sleep stage by the human expert. Let pi,j denote the proportion of samples in the i, jth cell of C, corresponding to ni,j: where n is the total number of the analyzed EEG segments. Furthermore, marginals pi+ and p+j can be defined by and
2.3.4.2. Kappa analysis.
and the chance agreement is defined as the estimate of Kappa is given by The variance of Kappa is as follows:
where
and ˆK can take values from the range [−1, 1]. However, positive values are expected since there should be a positive correlation between classifications performed by the human expert and LDA. Landis and Koch characterized the possible ranges for KHAT into three groups [52]: a value greater than 0.80 (i.e., >80%) represents strong agreement; a value between 0.40 and 0.80 (i.e., 40–80%) represents moderate agreement and a value below 0.40 (i.e., <40%) represents poor agreement. As it can be seen in Table 1, a strong agreement (ˆK = 0.85) between classifications performed by the human expert and LDA was found using the ΔD measure in the central channel of subject #16. For the particular confusion matrix presented in Table 1 with ˆK = 0.85, the corresponding overall accuracy was 90.37%. The fact that the ˆK statistic is asymptotically normally distributed provides a means for testing the significance of ˆK for a single confusion matrix to determine if the agreement between the classification performed between the human expert and LDA is significantly grater than zero, i.e., LDA performs better that than a random classifier. The test statistic is expressed by: Given the null hypothesis H0 : K = 0, and the alternative H1 : K ≠ 0, H0 is rejected if Z ≥ Zα/2, where α/2 is the confidence level of the two-tailed Z test. Moreover, there is a test to determine whether two confusion matrices are significantly different. This provide us the opportunity to compare classifications performed using different EEG features and channels. Let ˆK1 and ˆK2 ( var(ˆK1) and var(ˆK2) ) denote the estimates of the ˆK statistic (estimates of variances) for confusion matrices #1 and #2, respectively. The test statistic in this case is defined as
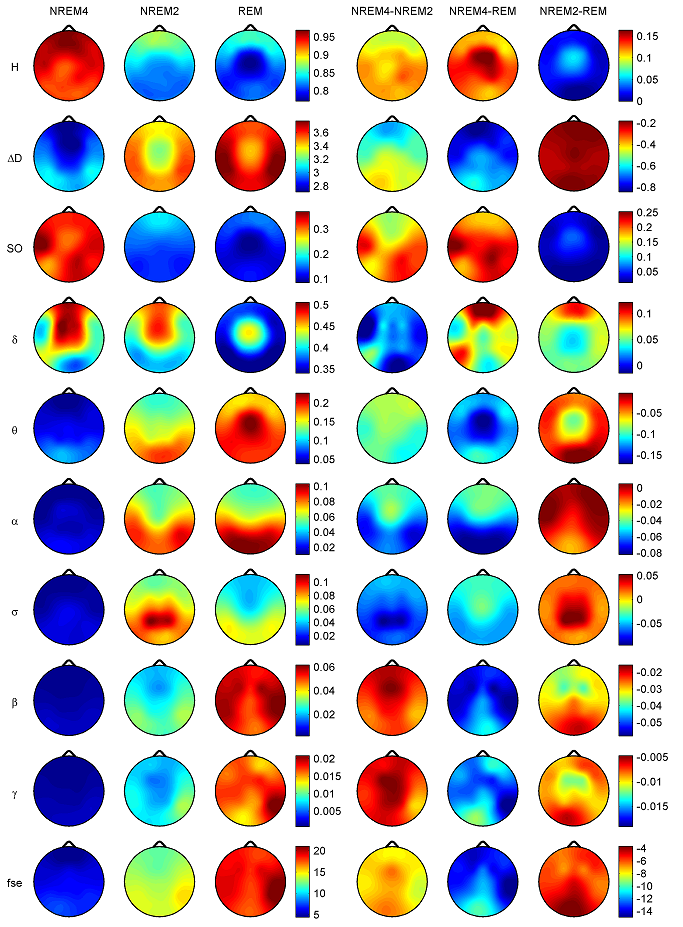
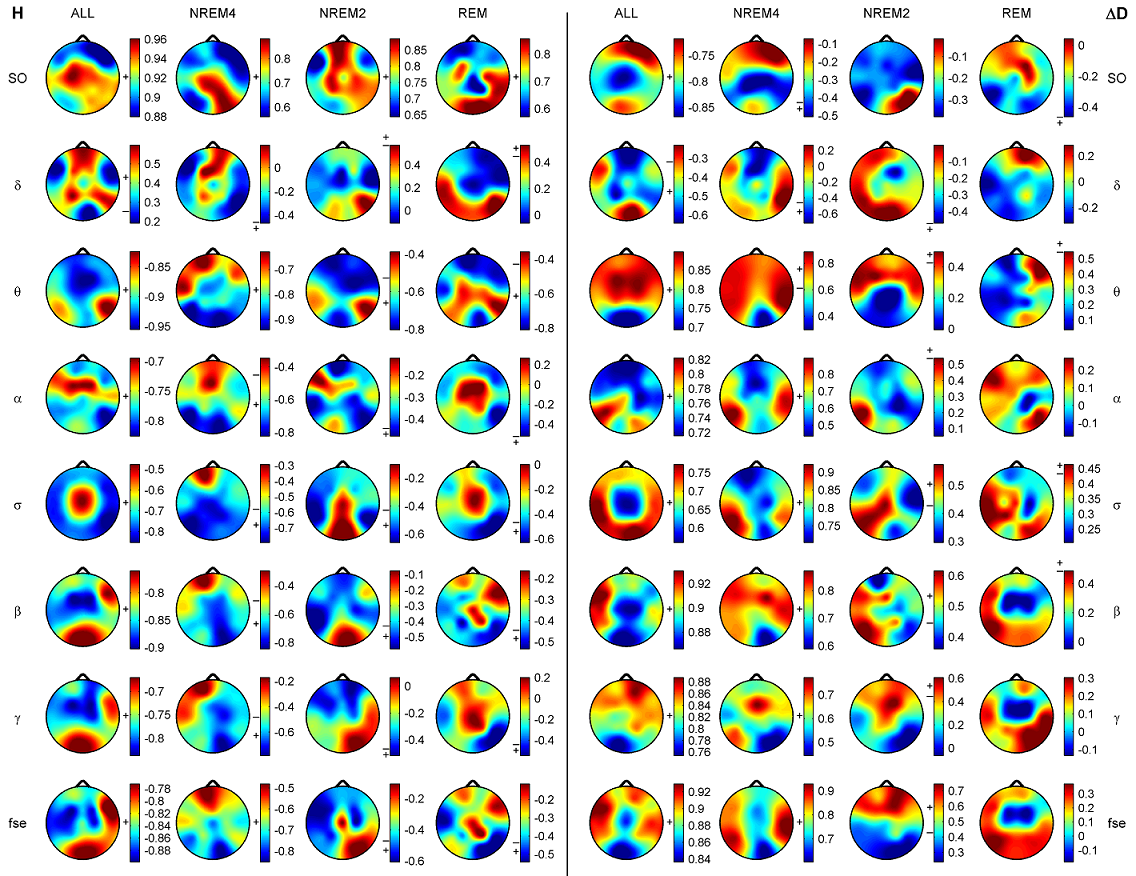
Z is standardized and normally distributed. Given the null hypothesisH0 : K1 −K2 =0, and the alternative H1 : K1 −K2 ≠ 0, H0 is rejected if Z ≥ Zα/2. respectively. The same comparison tests available for the Kappa coefficient apply to this conditional Kappa for an individual class. For more details and comparison of Kappa analysis to other methods we refer to [18,19]. 3. Results 3.1. Distribution of EEG measures across vigilance states and topographic locations Topographic distributions of group level medians for the three sleep stages as well as their differences are shown in Fig. 1. Results obtained for the fractal measures were in agreement with those presented in [95]. Namely, highest H values emerged frontally during all sleep stages, while the minimum was found during REM in the central zone. A HNREM4 >HNREM2 >HREM trend was present across the whole head surface. Measure ΔD, showed an opposite trend: DREM >DNREM2 >DNREM4. Minima of ΔD could be found in the fronto-central region during all sleep stages, while higher values were observed in the posterior circumferential channels. Salient H difference peaks that occurred for sleep stage pairs NREM4-REM and NREM2-REM could not be observed in the case of ΔD. Power spectral measures also exhibited expected topography. Relative band powers of slow activities (SO and δ bands) were higher for NREM4 than NREM2 as well as for NREM2 than REM. Generally, PSOr showed amore even topographic distribution when compared to Pδr in all sleep stages. Across all sleep stages the minimum of PSOr occurred at the vertex during REM sleep. Frontal regions exhibited slightly higher values of PSOr compared to other regions during NREM2 and REM. During NREM4 higher PSOr values occurred bilaterally in the fronto-temporal region and in C4, P4, O2 channels. Pδr exhibited higher values in the fronto-central channels during NREM4 and NREM2 and in the central region during REM sleep. Generally, faster activities (above 4 Hz) showed an opposite trend with lower relative band power values for deeper sleep stages. During NREM4 relatively even topographic distributions were found for faster activities. During NREM2 higher values appeared in the posterior region, showing maxima in the parietal channels for Pδr. REM sleep revealed a more diverse topography of faster activities. Maximum of theta activity was found centrally. Highest values in the α and σ bands occurred in posterior channels. Finally, relative power of β and γ frequency bands peaked in temporal channels. Spectral edge frequency showed lower values for deeper sleep stages as it was conjectured from Eq. (14). Slightly higher values of fse were present in posterior channels during NREM4 and NREM2, while during REM higher values were located in temporal channels. Comparing sleep stages using one-way Kruskal-Wallis ANOVA revealed highly significant (p < 0.00001) differences for all measures in all channels except for the relative power of the δ band. For detailed results see the supplementary Table S1. The level of significance for Pδr varied across channels between p < 0.01 and p < 0.00001. Pair-wise comparison of sleep stages using the rank post-hoc test resulted in most significant differences between sleep stages NREM4 and REM for all measures and channels with the exception of Pσr. This latter measure exhibited highest significance values between NREM4 and NREM2 in fronto-centroparietal channels (F3, Fz, F4, C3, Cz, C4, P3 and P4). In general, least or non-significant differences were observed between sleep stages NREM2 and REM. Comparison between NREM4 and NREM2 revealed significant values for all measures with the exception of the relative δ band power which reached significancy in F3, C3, P3 and P4 channels only.
Fig. 1. Topographic distribution of group level medians (medians of individual medians) for the analyzed sleep stages and differences of these medians between sleep stages. Relative band powers are denoted by the labels of the corresponding frequency bands. 3.1.1. Interhemispheric comparisons As expected, Wilcoxon matched pairs test revealed (Table 2) more significant differences for the non-symmetric Fz–Cz channel pair as compared with the other symmetric channel pairs. Out of the 30 cases (10 measures×3 stages) the Fz–Cz channel pair exhibited 19 significant values. Regarding the symmetric channel pairs most significant differences were found for channel pairs P3–P4 (10 cases) and O1–O2 (8 cases), while least significant differences appeared for the frontal channel pairs Fp1–Fp2 (2 cases), F7–F8 (4 cases) and F3–F4 (4 cases). In posterior channel pairs more significant results occurred during deeper sleep stages while in frontal channel pairs the least significant differences were found during sleep stage NREM4. Most significant interhemispheric differences occurred for relative powers of delta and beta frequency bands (7 cases for both out of total 24 = 3 sleep stages×8 symmetrical channel pairs). For Pδr significant cases were distributed similarly across sleep stages, while Pβr revealed the most (5 cases) significant differences for NREM2 sleep. PSOr resulted in 4 significant cases, all during NREM4. By contrast, no significant interhemispheric differences were found for Pγr in this sleep stage. No gross tendencies were observed for Pαr and Pσr across sleep stages and locations, however, there were some significant results. Compact EEG features (H,ΔD and fse) altogether revealed a lownumberof significant results: 5, 4 and 0 significant cases for NREM4, NREM2 and REM, respectively.
3.2. Cross-correlation analysis 3.2.1. Inter-site correlations
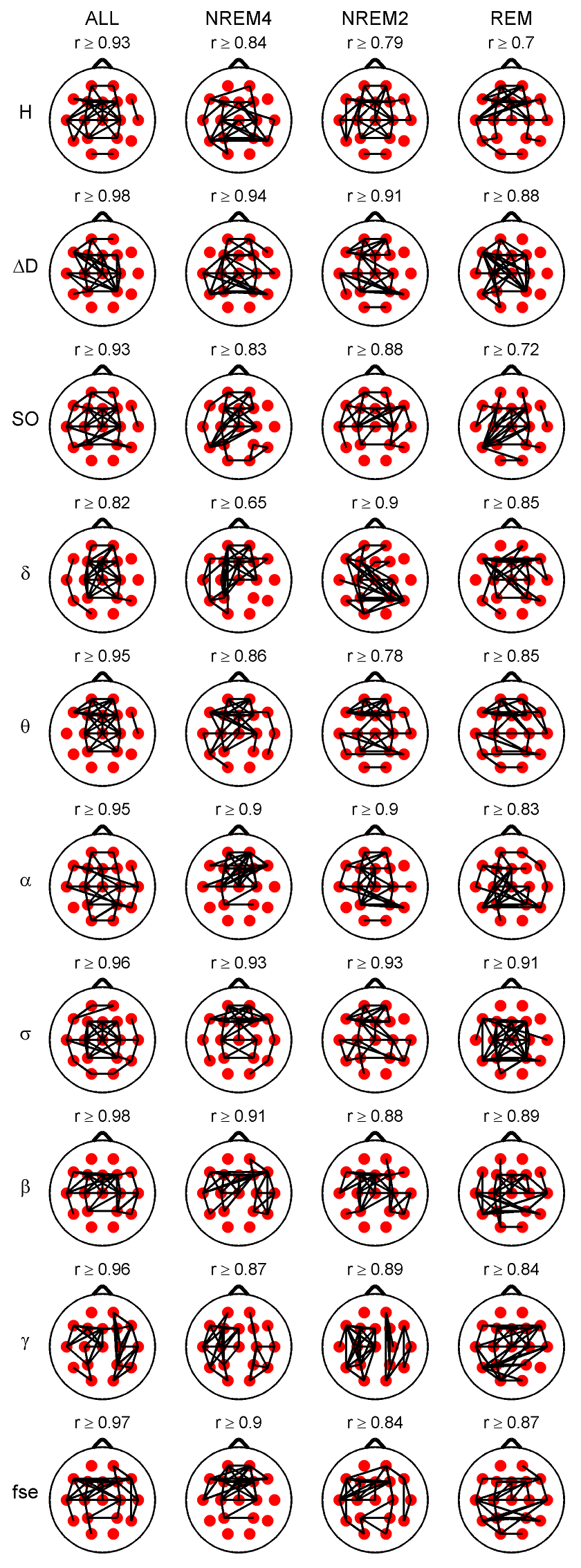
Fig. 2. Highest 35 inter-site correlations denoted by black lines drawn between the appropriate locations. Spearman’s correlation coefficients were calculated considering all sleep stages together (column ALL) as well as separately. Only significant (p < 0.05) correlations are depicted. Lowest presented correlation values can be found above the topographic maps. Relative band powers are denoted by the labels of the corresponding frequency bands. 3.2.2. Cross-correlation of measures 3.2.2.1. Cross-correlation between the fractal measures.
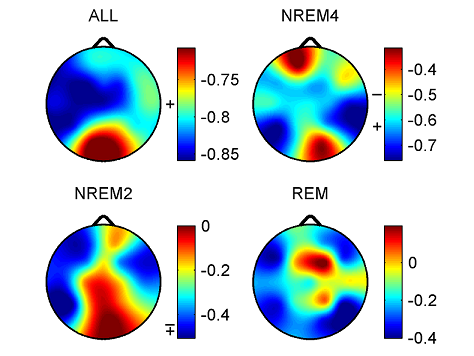
Fig. 3. Spearman cross-correlations between H and ΔD considering all sleep stages together as well as separately. Significant values (p < 0.05) are denoted on the left side of the color bars using the following notations: no sign (none of the values are significant); only + (all values are significant); + with a dash (only values below the dash are significant). 3.2.2.2. Cross-correlation between fractal and power spectral measures. Generally, correlations between ΔD and RBPs exhibited relationships with a sign opposite to that found for measure H. Namely, ΔD was negatively correlated with slow (SO and δ) while positively correlated with faster (above 4 Hz) activities. Similarly to H, more significant values appeared for deeper sleep stages. Compared to H, correlations of ΔD with slow activities were weaker and less significant. Highest positive correlation values between ΔD and PΘr were found in the anterior channels regardless of sleep depth. Relative power of alpha and sigma bands exhibited strongest positive correlations with ΔD in the temporal channels. Pβr and Pγr showed strongest positive correlations with ΔD around the Fz channel during NREM4, while the nadirs of these correlations were found during REM sleep in the same region. and statistics F(7, 58) = 200.99, p < 0.00001, Std . Err . Est . = 0.01, R = 0.98, R2 = 0.96, adjusted R2 = 0.96. For ΔD MLR was performed considering the T4 channel. The obtained result was with statistics F(7, 58) = 77.54, p < 0.00001, Std . Err . Est . = 0.12, R = 0.95, R2 = 0.9, adjusted R2 = 0.89. Finally, contribution of different frequency bands to the spectral edge frequency was tested in channel T6 with the result and the following statistics F(7, 58) = 360, p < 0.00001, Std . Err . Est . = 1.23, R = 0.99, R2 = 0.98, adjusted R2 = 0.97. Coefficients of relative band powers were standardized and their standard errors were provided in brackets. Only coefficients with stars above them were significant with notation: ** (p < 0.01), *** (p < 0.001), **** (p < 0.0001) and ***** (p < 0.00001).
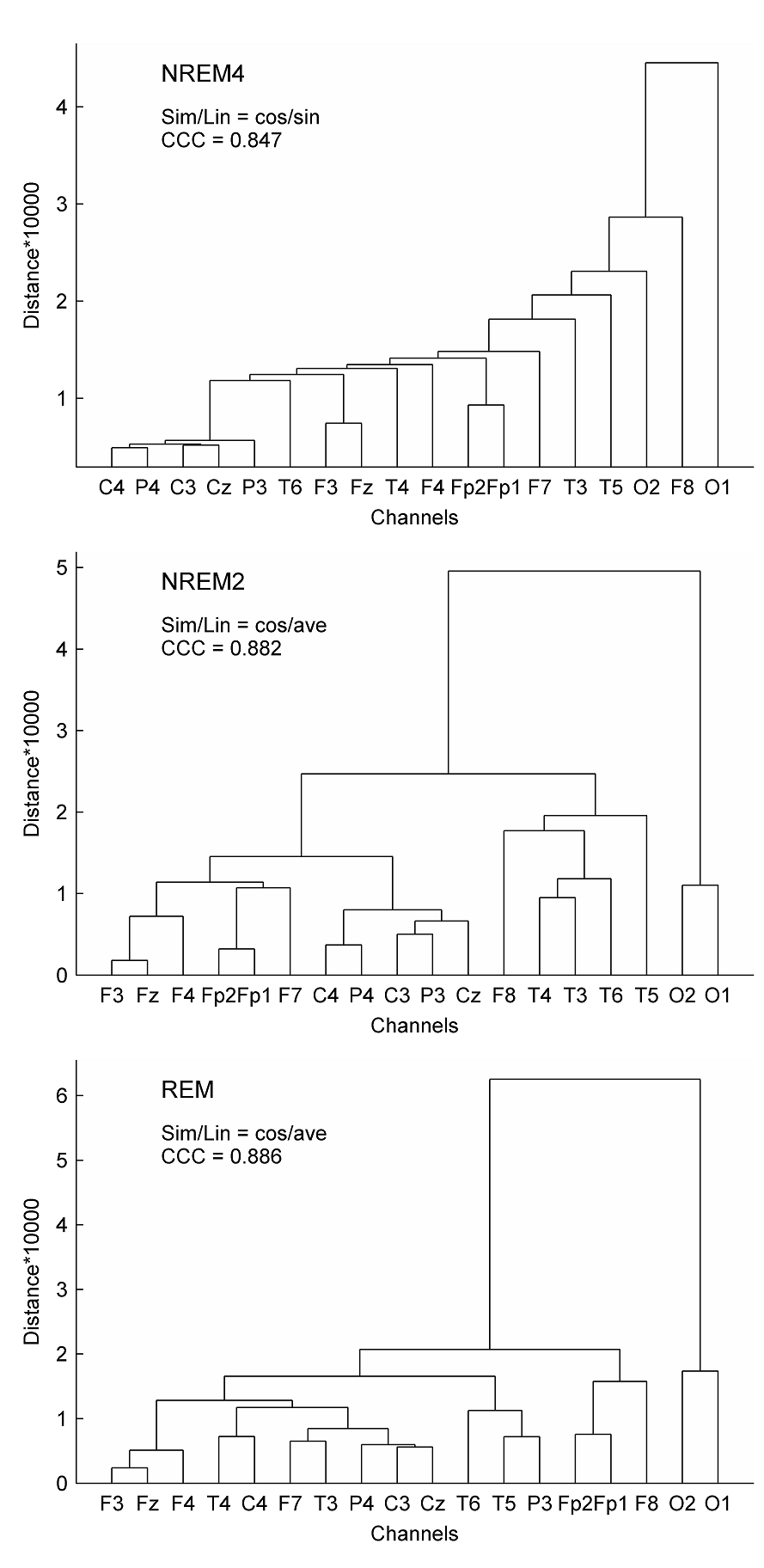
Fig. 4. Spearman cross-correlations between fractal and power spectral measures (PSMs). The left panel depicts results obtained for H. Cross-correlations between ΔD and PSMs are presented in the right panel. Sleep stages were considered together (columns denoted by ALL) as well as separately. Significant values (p < 0.05) are marked on the left side of the color bars using the following notations: no sign (none of the values are significant); only + (all values are significant); + with a dash (only values above/below the dash are significant). Relative band powers are denoted by the labels of the corr esponding frequency bands. 3.3. Clustering of channels and measures Hierarchical channel cluster trees were generated for all three sleep stages and all EEG features separately using the best similarity and linkage combinations. Visual inspection of the dendrograms revealed both common and distinct channel clusters across sleep stages (see e.g. Fig. 5 for measure ΔD). For statistical evaluation channels were clustered into 4 and 9 clusters, respectively (supplementary Table S2).
Fig. 5. Dendrograms of EEG channels obtained using the multifractal measure ΔD. Hierarchical cluster trees were generated applying the best similarity/linkage (Sim/Lin) methods according to the cophenet correlation coefficient (CCC). For the abbreviation of Sim/Lin methods see Section 2.3.3. As can be seen in Table S2 and Table 3, the 9-cluster analysis showed that most EEG features revealed proximity of symmetrical channels in the frontal region and during NREM4. As compared, during REM the symmetric channels were clustered together more posteriorly and circumferentially. Regardless of the sleep stage most of the measures (6–9 out of 10) indicated the proximity of F3–F4, C3–C4 and P3–P4 channels. The non-symmetrical Fz and Cz channels clustered together in much less cases. A more detailed examination of data in supplementary Table S2 unveiled that Fz tended to cluster with frontal F3 and F4 channels, while Cz mostly formed common clusters with the central channels C3 and C4. With regard to Pγr symmetric channels did not cluster together during NREM4 and NREM2 sleep stages but did so in REM sleep.
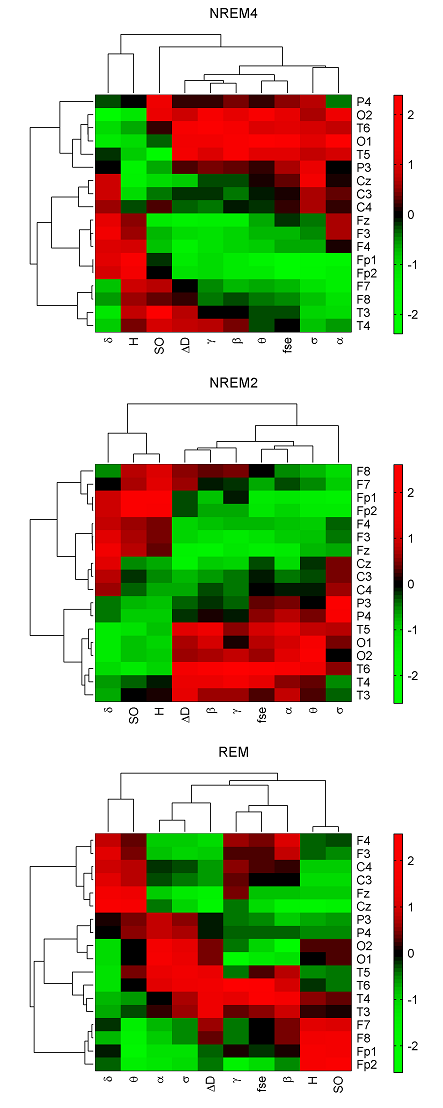
In the 4-cluster analysis, where all measures were considered separately, a rather uneven clustering of channels was found. Notably, in almost all cases there were channels (typically the circumferential ones such as: Fp1, Fp2, O1, O2, T5, T6) that formed individual clusters because of their large distance from the remaining EEG derivations. At the same time, several topographic features revealed by the above analyses could be verified. For example, highest Pσr values in parietal channels during NREM2 (Fig. 1) were reflected in a separate cluster formed by P3 and P4 channels. As another example disconnection of the hemispheres during NREM4 with regard to the relative power of the γ band (Fig. 2) was also supported by forming separate clusters over left and right hemispheres. Hierarchical clustering of channels was also carried out using all measures together to minimize the effect of “outlier” channels and to assess topography of overall brain dynamics. Performing 4-cluster analysis of hierarchical channel cluster trees (Fig. 6) revealed symmetric channel clusters for all sleep stages (Table 4). In general, separate clusters were formed by anterior, central, temporal and posterior channels. Topographic boundaries of these clusters slightly varied across sleep stages.
When combining the fractal measures only (Table 5) or the relative band powers only (Table 6) less symmetrical and slightly different topographic grouping of channels was obtained, e.g. the separate cluster that was formed for the parietal channels during NREM2 using all measures together (Table 4) was not found using the fractal measures only.
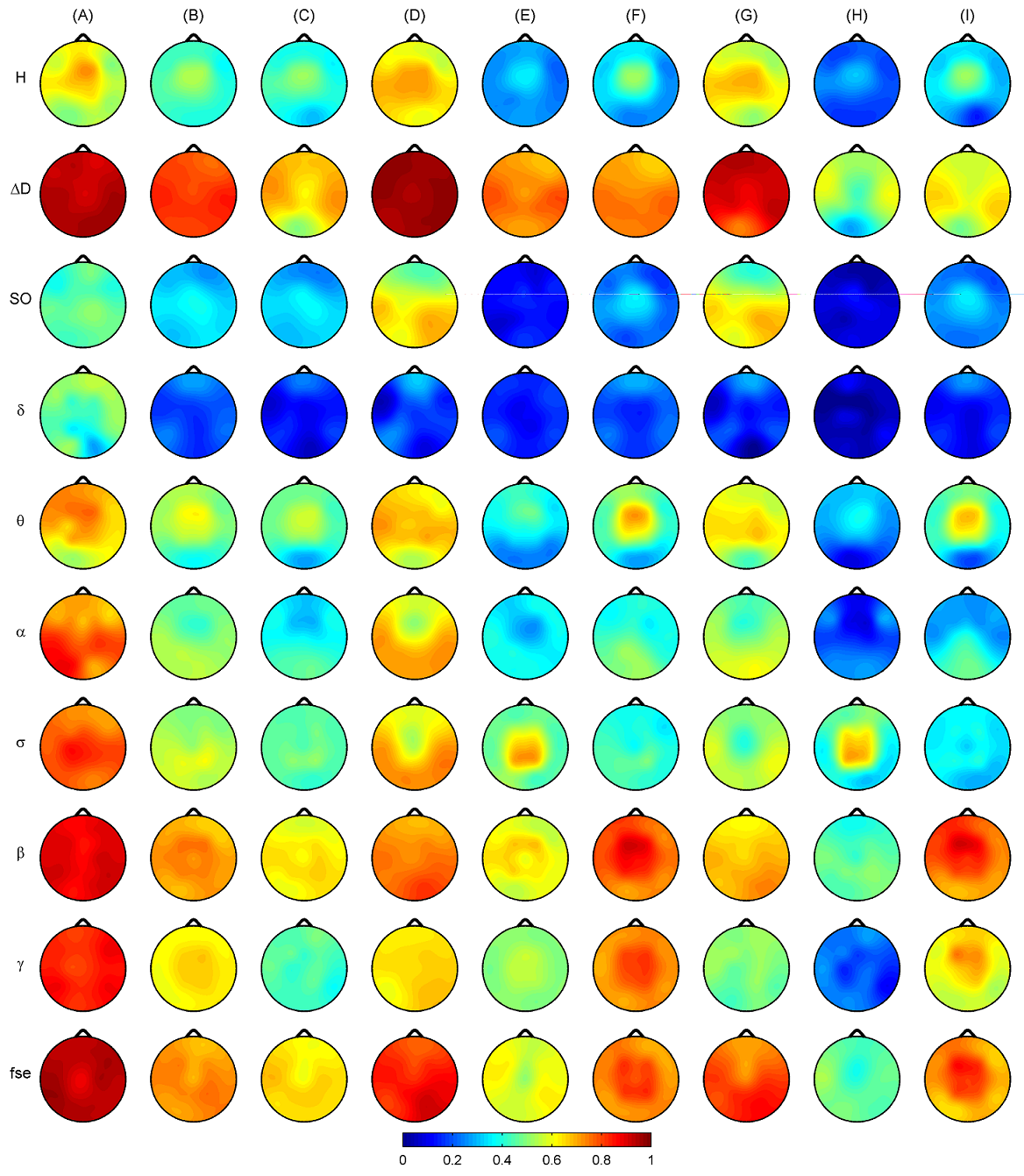
Fig. 6. Clustergrams generated for sleep stages NREM4, NREM2 and REM using group level medians of all measures in all channels. Heat maps and hereby the dendrograms were generated after z-standardization of the measures across the channels. Hierarchical channel cluster trees were generated using the cosine similarity metric and the unweighted average linkage method. The corresponding cophenet correlation coefficients (CCCs) were as follows: 0.749 (NREM4), 0.843 (NREM2) and 0.826 (REM). Measure dendrograms were constructed applying the Euclidean distance as a similarity measure and the unweighted average method for linkage (CCC: 0.959 (NREM4), 0974 (NREM2) and 0.886 (REM)). Relative band powers are denoted by the labels of the corresponding frequency bands. 3.4. Classification of sleep stages Wecarried out a sleep stage classification using LDA both at individual and group levels. Maximal ˆK values across subjects (Fig. 7A) yielded best classifications for ΔD, fse and Pβr features. There were 9 channels with highest ˆK values for ΔD and 8 channels for fse. Kappa analysis did not reveal significant differences between the performances of these two measures at either channel (Table 7, imax test). Taking the average of ˆK values across subjects, ΔD provided the best performance in all channels (Fig. 7B). At the group level (Fig. 7C) highest ˆK values were also found for ΔD for most of the channels (except for T5, P3, P4, O1 and O2 channels) followed by measures fse and Pβr. Comparing these three measures there were 8 channels (with a predominance of circumferential electrodes) where ΔD achieved significantly better performance compared to measures fse and Pβr (Table 7, g test). Considering the individual level conditional ˆK values averaged across subjects ΔD showed best performance for NREM4 (Fig. 7D) as well as for NREM2 (Fig. 7E) in all channels while during REM ΔD, Pβr, Pγr and fse revealed similar performance (Fig. 7F). Group level conditional ˆK values were presented in the last three columns of Fig. 7 (G, H, I). For NREM4 (Fig. 7G), ΔD showed the best classification results in all channels except for the occipital electrodes (Table 7, gcN4 test). During NREM2 best performance was also achieved for ΔD in most channels (13 channels) (Fig. 7H). Performance of ΔD significantly exceeded those of fse and Pβr in 9 channels (Table 7, gcN2 test). By contrast, during REM fse and Pβr significantly outperformed ΔD in all channels (Table 7, gcR test). Best classifications were obtained for measure Pβr in all channels. For all columns presented in Fig. 7 maximal ˆK values of ΔD were found in the circumferential channels (Table 8) with the best performance in T4 channel in 4 cases including mean ˆK values for the individual level (row B), ˆK values at the group level (row C) as well as conditional ˆK values in rows D and H. As can be seen in Table 8, best classification performance across EEG measures was found for ΔD considering all rows with the exception of rows (F, I) that is the conditional values for REM. In (rows A–C) best classifications of ΔD exceeded 80% (peaking in 97.78% at individual level for subject #3) considering the overall accuracy measure.
Fig. 7. Sleep stage classification results using linear discriminant analysis both at individual and group levels. (A) Maximal ˆK values taken across subjects. (B) Averaged individual ˆK values. (C) Group level ˆK values. (D) Individual conditional ˆK values averaged across subjects for sleep stage NREM4. (E) Individual conditional ˆK values averaged across subjects for sleep stage NREM2. (F) Individual conditional ˆK values averaged across subjects for REM sleep. (G) Group level conditional ˆK for NREM4 sleep. (H) Group level conditional ˆK for sleep stage NREM2. (I) Group level conditional ˆK for REM sleep. Relative band powers are denoted by the labels of the corresponding frequency bands.
4. Discussion To our knowledge this is the first study providing a detailed comparison of fractal and power spectral features of the human EEG considering the effects of topography and sleep stages. Fractality of EEG signals was assessed using both monofractal and multifractal measures. Power spectral properties were described by relative band powers and in amore compact way by estimating the spectral edge frequency. Sleep was analyzed considering sleep stages NREM4, NREM2 and REM separately as well as together. Topography was assessed with regard to functional connectivity by analyzing interhemispheric differences and regional clustering of EEG derivations. Our data indicate that despite of correlations between fractal and power spectral measures, fractal features carry additional information about EEG signals. Moreover, brain electrical activities are more complex than they could be fully described by a single monofractal exponent and therefore a multifractal approach may be more appropriate for modeling the fractal properties of brain dynamics. A main and novel finding of the study is that the overall sleep stage discrimination capability of the multifractal measure is superior compared to the relative band powers, the compact power spectral measure fse as well as the monofractal exponentH. Regarding overall accuracy ΔD exceeded 80% at the group level and achieved even 97.78% at the individual level. This finding speaks in favor of the individual level classification. Our results, in addition, indicate highest accuracy for the temporal channels which might surprise given that previous studies typically used central EEG channels for sleep stage classification. 4.1. Topographic and sleep stage-wise distribution of measures Topographic distribution of the analyzed measures (Fig. 1) was in general agreement with expectations. Results obtained for the fractal measures were completely in agreement with those findings published in [95]. Higher H values occurred for deeper sleep stages, while ΔD showed an opposite trend indicating that brain electrical activities tend to be less multifractal and to posses longer memory properties during deeper sleep stages. The decrease of ΔD with the deepening of sleep was opposite to the behavior of another multifractal measure, the range of singularity strength in a previous study [63] where the multifractality of EEG signals was assessed analyzing the distribution of zero-crossings. This discrepancy indicates a need for a systematic comparison of different estimation methods for multifractal analysis of EEG signals. The HNREM4 >HNREM2 >HREM trend was in agreement with results of previous studies that assessed the DFA λ exponent [53–55,86], the fractal exponent Κ [74] or the fractal dimension Df [73]. Namely, DFA exponent λ and κ increased with the deepening of sleep, while Df exhibited the opposite trend. This suggests that the Hurst exponent estimated based on R/S statistics might be able to reflect the self-similarity properties of the sleep EEG, although the aforementioned trend could not be observed for H in [1]. Nevertheless, searching for the direct relationship between exact values of fractal measures and physiological processes has less sense, because of the already mentioned doubts related to estimation of fractal measures from time series of finite length. Even classification of actual brain dynamics into one of two types of fractional time series (fGn and fBm) is questionable. According to one part of the studies, fGn behavior of the sleep EEG could be conjectured based on DFA λ exponent values below 1 [54,55] or estimated Hurst exponents below 1 using the R/S statistics approach such as in the present study and in [1,95]. Other studies, on the contrary, indicated fBm nature of the human sleep EEG by revealing DFA κ exponent values above 1 [53,86] or fractal exponent values in the range 1 <κ <3[74]. These discrepancies might be due to different estimation settings and indicate a need for a comprehensive comparison of different approaches used for the assessment of fractal properties, including classification of EEG signals into one of two classes of fractional time series (fGn or fBm) as it was proposed in [24–26]. Moreover, we should keep in mind that sleep EEG signals exhibit multifractal properties and thus monofractal analysis can only give a measure of the largest of their fractal dimensions. The 1/f noise-like power spectrum ofEEGsignals can be distorted during different conditions by characteristic peak frequencies (e.g. due to the alpha rhythm or intensive sleep spindles) that could destroy the self-similar nature of EEG. Possible analyses for such cases were proposed in [29,57]. Finally, we should also note that recent investigations revealed effects of gender and age [68] as well as genetic contributions to long-range temporal correlations [60] in wake EEG signals. Therefore, effects of these factors should be investigated during different sleep stages as well. 4.2. Cross-correlations between measures In our previous study [95] we found a tendency for an overall negative cross-correlation between H and ΔD. In that study we also revealed a specific topography of this relationship. Here we extend the characterization of the cross-correlation between H and ΔD by assessing sleep stages separately. Combining all sleep stages we found a strong negative correlation between H and ΔD with a nadir in the posterior channels (Fig. 3). As revealed by the sleep stage-wise analysis NREM2 and NREM4 contributed most to this occipital nadir. As compared to NREM4 weaker and less significant correlations emerged during NREM2. During REM there was a further weakening of correlations with a non-significant positive peak in the F3, Fz, F4 channels. 4.3. Interhemispheric differences and inter-site correlations In the present study we also compared inter-site correlations (Fig. 2) and interhemispheric differences of fractal and power spectral measures (Table 2). Surprisingly interhemispheric differences of specific measures varied with sleep stages and locations more than expected. In addition, it is difficult to relate these results to previous data where sleep stages were combined and/or EEG recordingwaslimited to a few channels. Nevertheless,wewere able to reveal some coherent tendencies regarding the interhemispheric differences of spectral powers, e.g.weobserved a right-hemisphere predominance of SO duringNREM4which might be related to those results by Sekimoto et al. [84] finding predominance of 0.5–2 Hz activity over the right hemisphere during all night sleep. At the same time delta activity on the left side tended to predominate in each sleep stage. During NREM4 and NREM2 we found higher theta activity on the right side, while during REM theta predominated on the left side corroborating the findings of Roth et al. [82]. The majority of interhemispheric comparisons of H and ΔD were not significant. Previous investigators revealed interhemispheric asymmetries for measures D2, L1 and Df considering C3–C4 channels [73] and interhemishperic differences for D2 in C3–C4, T3–T4 and O1–O2 locations [50]. Differences between earlier and the present data might be due to several methodological differences. Inter-site correlations of RBPs (Fig. 2) partially agreed with those results of coherence analyses revealed in [2,30,46]. Specifically, we found stronger interhemispheric Pγr correlations during REM compared to NREM4 and NREM2, a finding similar to obtained by Achermann and Borbély [2]. However, this result should be regarded with caution since disconnection of the hemispheres during NREM4 and NREM2 sleep regarding Pγr might also occur due to the application of two separate references [27,36,43].
Sleep stage discrimination capabilities of EEG features were tested in each channel both at individual and group levels (see Fig. 7 and Tables 7 and 8). Results indicate best overall classifications for ΔD followed by fse and Pβr. This could have been assumed from Table S1 since these three measures tended to reveal most significant differences for all sleep stage pairs. Superior performance of ΔD and fse is in agreement with results obtained previously for the entropy of amplitudes and spectral edge frequency measures [28]. In the present study best classifications for ΔD were revealed for the temporal channels. Measure H performed below the average considering all measures. This result is in contrast with a previous study [91] finding a superior performance of two other monofractal measures (the fractal exponent and the fractal dimension) when compared to spectral measures. To our surprise, worst classification performance was obtained for the relative band power of slow activities. This might be explained by confining the δ band to the 1- 4Hz range and considering slow oscillations (0.5–1 Hz) separately. The outstanding performance of ΔD, fse and Pβr indicate that it is the faster rather than the slow activities that might be of superior performance in the classification of sleep stages. As expected, better classifications were achieved at the individual level as compared to the group level, a finding that could be related to the considerable individual variability of sleep EEG features [23,32]. Conflict of interest The authors declare that they have no competing financial interests. Acknowledgment Appendix A. Supplementary data References [1] R. Acharya, O. Faust, N. Kannathal, T. Chua, S. Laxminarayan, Non-linear analysis of EEG signals at various sleep stages, Comput. Methods Progr. Biomed. 80 (1) (2005) 37–45. [2] P. Achermann, A. Borbély, Coherence analysis of the human sleep electroencephalogram, Neuroscience 85 (4) (1998) 1195–1208. [3] P. Achermann, A. Borbély, Low-frequency (<1 Hz) oscillations in the human sleep electroencephalogram, Neuroscience 81 (1) (1997) 213–222. [4] P. Bak, How Nature Works: The Science of Self-organized Criticality, Oxford University Press, Oxford, 1997. [5] P. Bak, C. Tang, K. Wiesenfeld, Self-organized criticality: an explanation of the 1/f noise, Phys. Rev. Lett. 59 (4) (1987) 381–384. [6] P. Bak, C. Tang, K. Wiesenfeld, Self-organized criticality, Phys Rev A 38 (1) (1988) 364–374. [7] A.L. Barabási, H.E. Stanley, Fractal Concepts in Surface Growth, Cambridge University Press, Cambridge, 1994. [8] J.B. Bassingthwaighte, L.S. Liebovitch, B.J. West, Fractal Physiology, Oxford University Press, New York, 1994. [9] C. Bédard, H. Kröger, A. Destexhe, Does the 1/f frequency scaling of brain signals reflect self-organized critical states? Phys. Rev. Lett. 97 (11) (2006) 118102. [10] J. Beggs, D. Plenz, Neuronal avalanches in neocortical circuits, J. Neurosci. 23 (35) (2003) 11167–11177. [11] O. Benoit, A. Daurat, J. Prado, Slow (0.7–2 Hz) and fast (2–4 Hz) delta components are differently correlated to theta, alpha and beta frequency bands during NREM sleep, Clin. Neurophysiol. 111 (12) (2000) 2103–2106. [12] J. Beran, Statistics for Long-Memory Processes, 1st ed., Chapman & Hall/CRC, New York, 1994. [13] T. Boji´ c, A. Vuckovic, A. Kalauzi, Modeling EEG fractal dimension changes in wake and drowsy states in humans—a preliminary study, J. Theor. Biol. 262 (2) (2010) 214–222. [14] R. Cerf, A. Daoudi, M. Ould Hénoune, E. el Ouasdad, Episodes of low-dimensional self-organized dynamics from electroencephalographic alpha-signals, Biol. Cybern. 77 (4) (1997) 235–245. [15] Z. Chen, K. Hu, P. Carpena, P. Bernaola-Galvan, H. Stanley, P. Ivanov, Effect of nonlinear filters on detrended fluctuation analysis, Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 71 (1 Pt 1) (2005) 011104. [16] Z. Chen, P. Ivanov, K. Hu, H. Stanley, Effect of nonstationarities on detrended fluctuation analysis, Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 65 (4 Pt 1) (2002) 041107. [17] D. Chialvo, P. Bak, Learning from mistakes, Neuroscience 90 (4) (1999) 1137–1148. [18] R.G. Congalton, K. Green, Assessing the Accuracy of Remotely Sensed Data: Principles and Practices, 2nd ed., CRC Press, Boca Raton, 2008. [19] P. Couto, Assessing the accuracy of spatial simulation models, Ecol. Modell. 167 (1–2) (2003) 181–198. [20] L. De Gennaro, M. Ferrara, Sleep spindles: an overview, Sleep Med. Rev. 7 (5) (2003) 423–440. [21] L. De Gennaro, M. Ferrara, G. Curcio, R. Cristiani, M. Bertini, Cortical EEG topography of REM onset: the posterior dominance of middle and high frequencies, Clin. Neurophysiol. 113 (4) (2002) 561–570. [22] L. De Gennaro, M. Ferrara, G. Curcio, R. Cristiani, Antero-posterior EEG changes during the wakefulness-sleep transition, Clin. Neurophysiol. 112 (10) (2001) 1901–1911. [23] L. De Gennaro, M. Ferrara, F. Vecchio, G. Curcio, M. Bertini, An electroencephalographic fingerprint of human sleep, Neuroimage 26 (1) (2005) 114–122. [24] D. Delignieres, S. Ramdani, L. Lemoine, K. Torre, M. Fortes, G. Ninot, Fractal analyses for ‘short’ time series: a re-assessment of classical methods, J. Math. Psychol. 50 (6) (2006) 525–544. [25] A. Eke, P. Herman, L. Kocsis, L. Kozak, Fractal characterization of complexity in temporal physiological signals, Physiol. Meas. 23 (1) (2002) R1–38. [26] A. Eke, P. Hermán, J. Bassingthwaighte, G. Raymond, D. Percival, M. Cannon, I. Balla, C. Ikrényi, Physiological time series: distinguishing fractal noises from motions, Pflugers Arch. 439 (4) (2000) 403–415. [27] M. Essl, P. Rappelsberger, EEG coherence and reference signals: experimental results and mathematical explanations, Med. Biol. Eng. Comput. 36 (4) (1998) 399–406. [28] J. Fell, J. Röschke, K. Mann, C. Schäffner, Discrimination of sleep stages: a comparison between spectral and nonlinear EEG measures, Electroencephalogr. Clin. Neurophysiol. 98 (5) (1996) 401–410. [29] T.C. Ferree, R.C. Hwa, Power-law scaling in human EEG: relation to Fourier power spectrum, Neurocomputing 52–54 (2003) 755–761. [30] R. Ferri, F. Rundo, O. Bruni, M. Terzano, C. Stam, Regional scalp EEG slowwave synchronization during sleep cyclic alternating pattern A1 subtypes, Neurosci. Lett. 404 (3) (2006) 352–357. [31] L. Finelli, A. Borbély, P. Achermann, Functional topography of the human nonREM sleep electroencephalogram, Eur. J. Neurosci. 13 (12) (2001) 2282–2290. [32] L. Finelli, P. Achermann, A. Borbély, Individual, ‘fingerprints’ in human sleep EEG topography, Neuropsychopharmacology 25 (5 Suppl.) (2001) S57–S62. [33] W. Freeman, L. Rogers, M. Holmes, D. Silbergeld, Spatial spectral analysis of human electrocorticograms including the alpha and gamma bands, J. Neurosci. Methods 95 (2) (2000) 111–121. [34] W. Freeman, M. Holmes, B. Burke, S. Vanhatalo, Spatial spectra of scalp EEG O. Jenni, E. van Reen, M. Carskadon, Regional differences of the sleep electroencephalogram and EMG from awake humans, Clin. Neurophysiol. 114 (6) (2003) 1053–1068. [35] J. González, A. Gamundi, R. Rial, M. Nicolau, L. de Vera, E. Pereda, Nonlinear, fractal, and spectral analysis of the EEG of lizard, Gallotia galloti, Am. J. Physiol. 277 (1 Pt 2) (1999) R86–R93. [36] S. Gudmundsson, T. Runarsson, S. Sigurdsson, G. Eiriksdottir, K. Johnsen, Reliability of quantitative EEG features, Clin. Neurophysiol. 118 (10) (2007) 2162–2171. [37] G. Gurman, Assessment of depth of general anesthesia. Observations on processed EEG and spectral edge frequency, Int. J. Clin. Monit. Comput. 11 (3) (1994) 185–189. [38] G. Gurman, S. Fajer, A. Porat, M. Schily, A. Pearlman, Use of EEG spectral edge as index of equipotency in a comparison of propofol and isoflurane for maintenance of general anaesthesia, Eur. J. Anaesthesiol. 11 (6) (1994) 443–448. [39] P. Halasz, K-complex, a reactive EEG graphoelement of NREM sleep: an old chap in a new garment, Sleep Med. Rev. 9 (5) (2005) 391–412. [40] S. Happe, P. Anderer, G. Gruber, G. Klösch, B. Saletu, J. Zeitlhofer, Scalp topography of the spontaneous K-complex and of delta-waves in human sleep, Brain Topogr. 15 (1) (2002) 43–49. [41] T. Higuchi, Relationship between the fractal dimension and the power law index for a time series: a numerical investigation, Physica D: Nonlin. Phenomena 46 (2) (1990) 254–264. [42] K. Hu, P. Ivanov, Z. Chen, P. Carpena, H. Stanley, Effect of trends on detrended fluctuation analysis, Phys. Rev. E: Stat. Nonlin. Soft Matter Phys. 64 (1 Pt 1) (2001) 011114. [43] S. Hu, M. Stead, Q. Dai, G. Worrell, On the recording reference contribution to EEG correlation, phase synchorony, and coherence, IEEE Trans. Syst. Man. Cybern. B Cybern. (2010). [44] H.E. Hurst, Long term storage capacity of reservoirs, Trans. Am. Soc. Civil Eng. 116 (1951) 770–799. [45] O. Jenni, E. van Reen, M. Carskadon, Regional differences of the sleep electroencephalogram in adolescents, J. Sleep Res. 14 (2) (2005) 141–147. [46] M. Kamin´ ski, K. Blinowska, W. Szclenberger, Topographic analysis of coherence and propagation of EEG activity during sleep and wakefulness, Electroencephalogr. Clin. Neurophysiol. 102 (3) (1997) 216–227. [47] H. Kantz, T. Schreiber, Nonlinear Time Series Analysis, 2nd ed., Cambridge University Press, Cambridge, New York and Melbourne, 2003. [48] S. Katsev, I. L’Heureux, Are Hurst exponents estimated from short or irregular time series meaningful? Comput. Geosci. (2003) 1085–1089. [49] V.G. Kiselev, K.R. Hahn, D.P. Auer, Is the brain cortex a fractal? Neuroimage 20 (3) (2003) 1765–1774. [50] T. Kobayashi, S. Madokoro, K. Misaki, J. Murayama, H. Nakagawa, Y. Wada, Interhemispheric differences of the correlation dimension in a human sleep electroencephalogram, Psychiatry Clin. Neurosci. 56 (3) (2002) 265–266. [51] V. Kulish, A. Sourin, O. Sourina, Human electroencephalograms seen as fractal time series: mathematical analysis and visualization, Comput. Biol. Med. 36 (3) (2006) 291–302. [52] J. Landis, G. Koch, The measurement of observer agreement for categorical data, Biometrics 33 (1) (1977) 159–174. [53] J.M. Lee, D.J. Kim, I.Y. Kim, K.S. Park, S.I. Kim, Nonlinear-analysis of human sleep EEG using detrended fluctuation analysis, Med. Eng. Phys. 26 (9) (2004) 773–776. [54] S. Leistedt, M. Dumont, N. Coumans, J. Lanquart, F. Jurysta, P. Linkowski, The modifications of the long-range temporal correlations of the sleep EEG due to major depressive episode disappear with the status of remission, Neuroscience 148 (3) (2007) 782–793. [55] S. Leistedt, M. Dumont, J.P. Lanquart, F. Jurysta, P. Linkowski, Characterization of the sleep EEG in acutely depressed men using detrended fluctuation analysis, Clin. Neurophysiol. 118 (4) (2007) 940–950. [56] C.D. Lewis, G.L. Gebber, P.D. Larsen, S.M. Barman, Long-term correlations in the spike trains of medullary sympathetic neurons, J. Neurophysiol. 85 (4) (2001) 1614–1622. [57] D. Lin, A. Sharif, H. Kwan, Scaling and organization of electroencephalographic background activity and alpha rhythm in healthy young adults, Biol. Cybern. 95 (5) (2006) 401–411. [58] K. Linkenkaer-Hansen, V. Nikouline, J. Palva, R. Ilmoniemi, Long-range temporal correlations and scaling behavior in human brain oscillations, J. Neurosci. 21 (4) (2001) 1370–1377. [59] K. Linkenkaer-Hansen, V. Nikulin, J. Palva, K. Kaila, R. Ilmoniemi, Stimulusinduced change in long-range temporal correlations and scaling behaviour of sensorimotor oscillations, Eur. J. Neurosci. 19 (1) (2004) 203–211. [60] K. Linkenkaer-Hansen, D. Smit, A. Barkil, T. van Beijsterveldt, A. Brussaard, D. Boomsma, A. van Ooyen, E. de Geus, Genetic contributions to long-range temporal correlations in ongoing oscillations, J. Neurosci. 27 (50) (2007) 13882–13889. [61] C. Long, N. Shah, C. Loughlin, J. Spydell, R. Bedford, A comparison of EEG determinants of near-awakening from isoflurane and fentanyl anesthesia. Spectral edge, median power frequency, and delta ratio, Anesth. Analg. 69 (2) (1989) 169–173. [62] S.B. Lowen, L.S. Liebovitch, J.A. White, Fractal ion-channel behavior generates fractal firing patterns in neuronal models, Phys. Rev. E Stat. Phys. Plasmas Fluids Relat. Interdiscip. Top. 59 (5 Pt B) (1999) 5970–5980. [63] Q.L. Ma, X.B. Ning, J. Wang, C.H. Bian, A new measure to characterize multifractality of sleep electroencephalogram, Chin. Sci. Bull. 51 (24) (2006) 3059–3064. [64] B.B. Mandelbrot, M.S. Taqqu, Robust R/S analysis of long-run serial correlation, in: Proceedings of the 42nd Session of the International Statistical Institute, 48, Book 2, Manila, 1979, pp. 69–104. [65] B.B. Mandelbrot, The Fractal Geometry of Nature, 1st ed., W.H. Freeman and Company, New York, 1982. [66] M. Massimini, R. Huber, F. Ferrarelli, S. Hill, G. Tononi, The sleep slow oscillation as a traveling wave, J. Neurosci. 24 (31) (2004) 6862–6870. [67] V. Nikulin, T. Brismar, Long-range temporal correlations in alpha and beta osc illations: effect of arousal level and test-retest reliability, Clin. Neurophysiol. 115 (8) (2004) 1896–1908. [68] V. Nikulin, T. Brismar, Long-range temporal correlations in electroencephalographic oscillations: Relation to topography, frequency band, age and gender, Neuroscience 130 (2) (2005) 549–558. [69] M. Palus, Nonlinearity in normal human EEG: cycles, temporal asymmetry, nonstationarity and randomness, not chaos, Biol. Cybern. 75 (5) (1996) 389–396. [70] A. Pellionisz, Neural geometry: towards a fractal model of neurons, in: R.M.J. Cotterill (Ed.), Models of Brain Function, Cambridge University Press, Cambridge, New York and Melbourne, 1989, pp. 453–464. [71] C. Peng, S. Havlin, H. Stanley, A. Goldberger, Quantification of scaling exponents and crossover phenomena in nonstationary heartbeat time series, Chaos 5 (1) (1995) 82–87. [72] C.K. Peng, S.V. Buldyrev, S. Havlin, M. Simons, H.E. Stanley, A.L. Goldberger, Mosaic organization of DNA nucleotides, Phys. Rev. E 49 (2) (1994) 1685. [73] E. Pereda, A. Gamundi, M. Nicolau, R. Rial, J. González, Interhemispheric differences in awake and sleep human EEG: a comparison between non-linear and spectral measures, Neurosci. Lett. 263 (1) (1999) 37–40. [74] E. Pereda, A. Gamundi, R. Rial, J. González, Non-linear behaviour of human EEG: fractal exponent versus correlation dimension in awake and sleep stages, Neurosci. Lett. 250 (2) (1998) 91–94. [75] L. Poupard, R. Sartčne, J. Wallet, Scaling behavior in beta-wave amplitude modulation and its relationship to alertness, Biol. Cybern. 85 (1) (2001) 19–26. [76] G. Rangarajan, M. Ding, Integrated approach to the assessment of long range correlation in time series data, Phys. Rev. E Stat. Phys. Plasmas Fluids Relat. Interdiscip. Top. 61 (5A) (2000) 4991–5001. [77] P. Rapp, A. Albano, T. Schmah, L. Farwell, Filtered noise can mimic lowdimensional chaotic attractors, Phys. Rev. E Stat. Phys. Plasmas Fluids Relat. Interdiscip. Top. 47 (4) (1993) 2289–2297. [78] A. Rechtschaffen, A. Kales, Amanual of standardized terminology, techniques and scoring system for sleep stages of human subjects, Bethesda, MD, U.S. National Institute of Neurological Diseases and Blindness, Neurol. Inform. Netw. (1968). [79] A. Rényi, Probability Theory, North Holland, Amsterdam, 1971. [80] P.A. Robinson, C.J. Rennie, J.J. Wright, H. Bahramali, E. Gordon, D.L. Rowe, Prediction of electroencephalographic spectra from neurophysiology, Phys. Rev. E 63 (2) (2001) 021903. [81] P.A. Robinson, C.J. Rennie, D.L. Rowe, Dynamics of large-scale brain activity in normal arousal states and epileptic seizures, Phys. Rev. E 65 (4) (2002) 041924. [82] C. Roth, P. Achermann, A. Borbély, Frequency and state specific hemispheric asymmetries in the human sleep EEG, Neurosci. Lett. 271 (3) (1999) 139–142. [83] T. Schreiber, A. Schmitz, Surrogate time series, Physica D: Nonlin. Phenom. 142 (3–4) (2000) 346–382. [84] M. Sekimoto, M. Kato, N. Kajimura, T. Watanabe, K. Takahashi, T. Okuma, Asymmetric interhemispheric delta waves during all-night sleep in humans, Clin. Neurophysiol. 111 (5) (2000) 924–928. [85] M. Shadlen, W. Newsome, The variable discharge of cortical neurons: implications for connectivity, computation, and information coding, J. Neurosci. 18 (10) (1998) 3870–3896. [86] Y. Shen, E. Olbrich, P. Achermann, P.F. Meier, Dimensional complexity and spectral properties of the human sleep EEG, Clin. Neurophysiol. 114 (2) (2003) 199–209. [87] Y. Shu, A. Hasenstaub, D. McCormick, Turning on and off recurrent balanced cortical activity, Nature 423 (6937) (2003) 288–293. [88] S. Spasic, A. Kalauzi, G. Grbic, L. Martac, M. Culic, Fractal analysis of rat brain activity after injury, Med. Biol. Eng. Comput. 43 (3) (2005) 345–348. [89] C. Stam, J. Pijn, P. Suffczynski, F. Lopes da Silva, Dynamics of the human alpha rhythm: evidence for non-linearity? Clin. Neurophysiol. 110 (10) (1999) 1801–1813. [90] C. Stam, Nonlinear dynamical analysis of EEG and MEG: review of an emerging field, Clin. Neurophysiol. 116 (10) (2005) 2266–2301. [91] K. Susmáková, A. Krakovská, Discrimination ability of individual measures used in sleep stages classification, Artif. Intell. Med. 44 (3) (2008) 261–277. [92] G. Tinguely, L.A. Finelli, H.P. Landolt, A.A. Borbely, P. Achermann, Functional EEGtopography in sleep and waking: state-dependent and state-independent features, Neuroimage 32 (1) (2006) 283–292. [93] V. Vyazovskiy, P. Achermann, A. Borbély, I. Tobler, Interhemispheric coherence of the sleep electroencephalogram in mice with congenital callosal dysgenesis, Neuroscience 124 (2) (2004) 481–488. [94] V. Vyazovskiy, I. Tobler, Regional differences in NREM sleep slow-wave activity in mice with congenital callosal dysgenesis, J. Sleep Res. 14 (3) (2005) 299–304. [95] B. Weiss, Z. Clemens, R. Bódizs, Z. Vágó, P. Halász, Spatio-temporal analysis of monofractal and multifractal properties of the human sleep EEG, J. Neurosci. Methods 185 (1) (2009) 116–124. [96] E. Werth, P. Achermann, A. Borbély, Brain topography of thehumansleep EEG: antero-posterior shifts of spectral power, Neuroreport 8 (1) (1996) 123–127. [97] E. Werth, P. Achermann, A. Borbély, Fronto-occipital EEG power gradients in human sleep, J. Sleep Res. 6 (2) (1997) 102–112. [98] E. Werth, P. Achermann, D. Dijk, A. Borbély, Spindle frequency activity in the sleep EEG: individual differences and topographic distribution, Electroencephalogr. Clin. Neurophysiol. 103 (5) (1997) 535–542. [99] A. Yasoshima, H. Hayashi, S. Iijima, Y. Sugita, Y. Teshima, T. Shimizu, Y. Hishikawa, Potential distribution of vertex sharp wave and saw-toothed wave on the scalp, Electroencephalogr. Clin. Neurophysiol. 58 (1) (1984) 73–76. [100] J. Zeitlhofer, G. Gruber, P. Anderer, S. Asenbaum, P. Schimicek, B. Saletu, Topographic distribution of sleep spindles in young healthy subjects, J. Sleep Res.6 (3) (1997) 149–155.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||