|
Corresponding author: Anna Szűcs, MD, National Institute of Psychiatry and Neurology, Hűvösvölgyi út 116, H–1021 Budapest (Hungary), Fax +36 1 391 5438, E-Mail szucsan@bakats.tvnet.hu National Institute of Psychiatry and Neurology, Budapest, Hungary Insomnia is traditionally placed on the border between psychiatry and general health care. Only 1.6% of insomnia cases are related to neurological disorders such as parkinsonism, dementia, sleep-related epilepsy, fatal familial insomnia and sleep-related headaches [1]. Diseases affecting sleep-initiating structures cause insomnia, e.g. lesions of the pontine tegmentum and the thalamus [2]. Although the sleep-regulatory function of the basal forebrain is well known in animal models, there are no clinical reports in this respect, except for classic von Economo encephalitis affecting the anterior hypothalamus and causing persistent insomnia [3]. Case Report
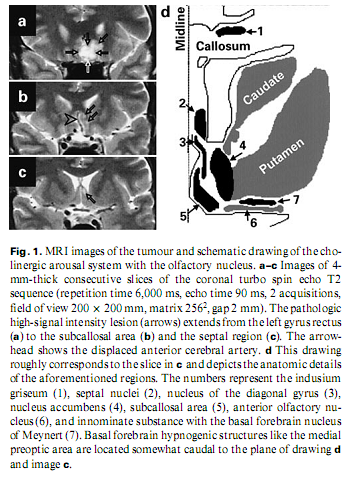
A 53-year-old engineer presented with insomnia for the previous 3 months. He reported that he had not been sleeping for 4–5 weeks, was depressive, inactive and helpless. He thought he was being poisoned by sleeping pills and was afraid of having cancer. There were no physical signs or vegetative abnormalities. Laboratory data, X-ray of the chest, carotid sonography and ultrasound of the abdomen were negative. An EEG revealed scarce bifrontal theta waves. Psychological tests showed regression and schizophreniform and moderate organic deterioration signs; neuro-psychological testing revealed no deficit. Native and contrast-enhanced MRI detected a space-occupying lesion of 25 x 20 x 20 mm in the posterior part of the left gyrus rectus extending to the subcallosal area and the septal region, displacing the anterior cerebral artery. It had an inhomogeneous signal, low on T1- and high on T2-weighted and FLAIR images with no contrast enhancement (fig. 1). On MRI repeated after 4 months, the lesion was unchanged, and it was presumed to be a low-grade glioma or a dysgenetic tumour.
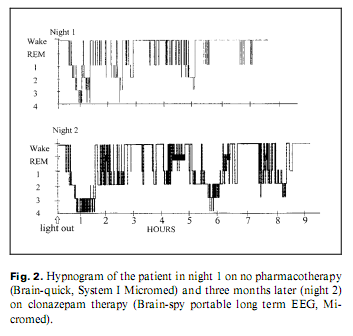
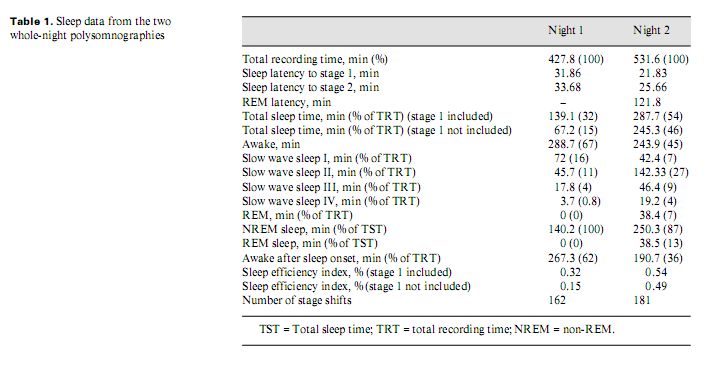
Because of his paranoia at first the patient did not accept pharmacotherapy; later, he took clonazepam and slept better. He refused neurosurgical intervention. After 8–9 months, his psychopathological and sleep state improved. Two whole-night polysomnographies were performed, one (Brain-quick, System 1 Micromed) on no treatment (night 1) and one (Brain-spy portable long-term EEG, Micromed) 8 months later, on clonazepam therapy (night 2) (table 1). His sleep was fragmented with short deep slow wave sleep and no (night 1) or little (night 2) rapid eye movement (REM) sleep (fig. 2). Analysing the micro-structure of sleep, we calculated different types of K complexes [4]: K complex standing alone directly followed by alpha activity for 0.5–5.0 s (K-alpha), delta waves (K-delta) and sleep spindles (K-sigma). Comparing the frequency of K complexes to reference values [5], it was high in stage 1 during both nights. K-alpha complexes were surprisingly frequent during both nights: more than 1/2 and 1/3 of the different types altogether were K-alpha complexes in night 1 and 2, respectively, predominating stage 1 sleep. Frequent awakenings were nearly always preceded by a K-alpha complex. The cyclic alternating pattern (CAP) represents relative arousal within non-REM sleep. The CAP rate (the time of CAP/total sleep time) in middle-aged persons is 38.2–42.7% [6]; in our case, it was 70 and 63%, in night 1 and 2, respectively. No periodic leg movements and no pathologic sleep apnoea/hypopnoea index were found.
DiscussionAnimal studies have revealed that the medial preoptic/anterior hypothalamic (basal forebrain) region, the orbito-frontal cortex and the lateral olfactory tubercle have hypnogenic functions [7–9]. Overlapping them there is the basal forebrain cholinergic arousal system –the medial septal nuclei, the vertical and horizontal limbs of the diagonal bands of Broca, the magnocellular preoptic area, the substantia innominata and the nucleus basalis of Meynert [7]. According to functional studies, lesion of these structures is associated also with psychopathology [10]. These small structures cannot be identified on MRI images, but anatomical landmarks indicate their normal location. The lesion in our case probably invaded the basal forebrain cholinergic system (septal nuclei, nucleus and diagonal band of Broca, innominate substance with the basal forebrain nucleus of Meynert and the preoptic area). The hypnogenic areas were not directly involved but could have been compressed (fig. 1). The patient’s insomnia is reflected both in classical and microstructural sleep characteristics; i.e. frequent K complexes in stage 1, the unconventional K-alpha complexes reflecting increased arousal level [4] and a high CAP rate, signalling instability of sleep. It seems reasonable to presume that this insomnia has an organic origin, i.e. that the tumour has damaged sleep-inducing structures or irritated arousal structures. It may be responsible also for the concomitant psychopathological syndrome associated with the patient’s insomnia. REM sleep loss is probably caused by increased arousal. We conclude that insomnia may be a focal symptom of the basal forebrain tumour. AcknowledgmentsWe are grateful to Zita Csepella for assistance in sleep scoring, and to Gábor Szabó and Levente Liszka for technical assistance. References1 Buysse DJ, Reynolds CF 3rd, Kupfer DJ, Thorpy MJ, Bixler E, Manfredi R, Kales A, Vgontzas A, Stepanski E, Roth T, et al: Clinical diagnosis in 216 insomnia patients using the International Classification of Sleep Disorders (ICSD), DSM-IV and ICD-10 categories: A report from the APA/NIMH DSM-IV Field Trial. Sleep 1994;17:630–637. 2 Culebras A: Neuroanatomic and neurologic correlates of sleep disturbances. Neurology 1992;42(7 suppl 6):19–27. 3 von Economo C: Sleep as a problem of localization. J Nerv Ment Dis 1930;71:249–259. 4 Halász P: Hierarchy of micro-arousals and the microstructure of sleep. Neurophysiol Clin 1998;28:461–475 5 Kubicki S, Herrmann WM: The future of computer-assisted investigation of the polysomnogram: Sleep microstructure. J Clin Neurophysiol 1996;13:285–294. 6 Terzano MG, Parrino L: Origin and significance of the cyclic alternating pattern (CAP). Sleep Med Rev 2000;4:101–123. 7 Szymusiak R: Magnocellular nuclei of the basal forebrain: Substrates of sleep and arousal regulation. Sleep 1995;18:478–500. 8 Siegel J, Wang RY: Electroencephalographic, behavioral, and single-unit effects produced by stimulation of forebrain inhibitory structures in cats.Exp Neurol 1974;42:28–50. 9 Obál F Jr, Benedek G, Réti G, Obál F: Tonic hypnogenic effect of the olfactory tubercle. Exp Neurol 1980;69:202–208. 10 Heimer L: Basal forebrain in the context of schizophrenia. Brain Res Brain Res Rev 2000;31:205–235
|