Corresponding author: Róbert Bódizs, Institute of Behavioural Sciences, Semmelweis University, Nagyvárad tér 4, H-1089 Budapest, Hungary (e-mail: bodrob@net.sote.hu )
ABSTRACT
Our study intends to examine whether the social brain theory is applicable to human individual differences. According to the social brain theory primates have larger brains as it could be expected from their body sizes due to the adaptation to a more complex social life. Regarding humans there were few studies about the relationship between theory of mind and frontal and temporal brain lobes. We hypothesized that these brain lobes, as well as the whole cerebrum and neocortex are in connection with the Sociability personality dimension that is associated with individuals’ social lives. Our findings support this hypothesis as Sociability correlated positively with the examined brain structures if we control the effects of body size differences and age. These results suggest that the social brain theory can be extended to human interindividual differences and they have some implications to personality psychology too.
Keywords
social brain theory, sociability, brain size, MRI, ZKPQ
Introduction
It is well-known that among vertebrates, primates have larger brains than it is expected from their body sizes. The social brain theory provides a plausible explanation for this phenomenon. According to this hypothesis, the unusual brain size is due to the adaptation to a more complex social life (Barton and Dunbar, 1997; Byrne and Whiten, 1988; Dunbar, 1998), however, the exact social skills that resulted in this have not been clarified yet. Many authors provide a reflection on the social brain theory at a behavioral level (Byrne and Whiten, 1988; Dunbar and Shultz, 2007a, 2007b; Kudo and Dunbar, 2001; Shultz and Dunbar, 2007), but these studies concentrate on interspecies differences. Social complexity requires large cognitive capacity. To live in a stable social group and sustain group cohesion members have to coordinate their behavior with others (Dunbar and Shultz, 2007b; Shultz and Dunbar, 2007), as well as to manage complex relational information (Dunbar, 1998). Individuals have to maintain more relationships and be able to respond appropriately to social interactions. Individuals have different goals and desires that might not conflict with those of others (Barrett, Henzi, and Dunbar, 2003). The authors suggest that “the ability to reason causally, the ability to reason analogically, the ability to exert cognitive control to generate and assess alternative options, and the ability to formulate these options into alternative sequences of future actions and to select between them” (pp. 496) are critical to develop high-level sociocognitive abilities.
The social brain theory is supported by plenty of quantitative research on primates and some other non-primate (carnivores and ungulates) mammals (Dunbar and Shultz, 2007a). According to the review of Dunbar and Shultz (2007b), correlations were observed between relative brain size or neocortex size and variables that indicate social complexity. These variables are: social group size (Dunbar, 1992), number of females in the group (Lindenfors, 2005), grooming clique size (Kudo and Dunbar, 2001), the frequency of coalitions (Dunbar and Shultz, 2007a), male mating strategies (Pawlowski, Lowen and Dunbar, 1998), the prevalence of social play (Lewis, 2001), the frequency of tactical deception (Byrne and Corp, 2004), and the frequency of social learning (Reader and Laland, 2002). Shultz and Dunbar (2007) have demonstrated that pairbonded species of vertebrates have larger brains for body size than species with other types of mating system. Moreover, in primates the quantitative group size has a significant effect on brain size, but in other taxa does not.
Fewer studies have been carried out on humans. Dunbar, McAdam and O‟Connell (2005) have shown that relative frontal lobe volume is associated with the speed with which individuals solve a puzzle box task comparing chimpanzees, orangutans and children. Stiller and Dunbar (2007) studied the relationship between social network size and two cognitive processes. Perspective taking competence was related to the size of the individual’s support clique (the number of individuals in the innermost circle of friends), while the short time memory performance was associated to the sympathy group size, which is a larger network of 12-20 people who are contacted in every month. Powell and colleagues (2010) found significant relationship between the orbital prefrontal cortex volume and intentional competence. According to the reviews of Gallagher and Frith (2003) and Abu-Akel (2003), theory of mind can be connected to the anterior paracingulate cortex, the anterior cingulate gyrus, the ventral and medial prefrontal cortex, the inferolateral frontal cortex, the superior temporal sulcus, the temporal poles, and the amygdala too besides the orbitofrontal cortex. Furthermore, Dunbar (2009) cites two highly relevant unpublished results. By using fMR I Birch, Lewis, Dunbar (unpublished, as cited in Dunbar 2009) observed that tasks in which participants had to represent the mind states of other individuals were cognitively more demanding. In other studies using voxel-based morphometry, theory of mind correlated with the volumes of the frontal and the temporal lobes (Lewis et al., unpublished, as cited in Dunbar 2009).
Furthermore, indirect information on the possible associations of brain size and social life among humans is provided by the studies examining neuroanatomical correlates of the levels of sociability-related personality dimensions. Extraversion is a trait with potential relevance expressing the individual differences in sociability and on which neuroanatomical investigations have carried out. In these studies, the Extraversion factor of NEO-PI-R (Revised Neuroticism-Extroversion-Openness Personality Inventory) or NEO-FFI (NEO-Five Factor Inventory, which is the short version of NEO-PI-R) was used. The self-report inventories were developed by Costa and McCrae (Costa, and McCrae, 1992; Costa, McCrae, and Dye, 1991) for measuring the factors of the Big Five. The Extraversion dimension has six subdomains: Warmth, Gregariousness, Assertiveness, Activity, Excitement Seeking, and Positive Emotion. Omura, Constable, and Canli (2005) employed the method called voxel based morphometry which technique can objectively measure gray matter volume and concentration. The authors reported positive correlations of the Extraversion factor of NEO-PI-R with the left amygdala and the bilateral orbitofrontal cortex, but negative correlations with the bilateral precentral gyrus. Other methods were used by Wright and colleagues (2006), who measured cortical thickness and the volume of amygdala by manual tracing. No significant correlations between Extraversion (assessed by the NEO-FFI) and the amygdala volume were observed. Moreover, in contrast with Omura, Constable, and Canli (2005) Wright and colleagues (2006) found significant negative correlations between Extraversion and the thickness of the right inferior prefrontal (BA 45) and the middle frontal cortices (BA 9), as well as of the right fusiform gyrus (BA 20). The authors suggested that their results are consistent with two classical biological models of personality (Eysenck‟s and Gray‟s) that claim that extraverts have lower cortical activity than introverts. Extraverts have thinner cortex which may be associated with lower metabolic activity. Knutson and colleagues (2001) examined the relationship between the ratio of the brain to the remainder of the intracranial volume and the NEO-PI-R factors, but correlation with Extraversion was not observed. Because of using the NEO-PI-R or NEO-FFI, the Extraversion factor is more than pure sociability (Sociability is one of its facets), other facets are for example Activity, which is a separate trait in Zuckerman‟s model, Sensation Seeking which is a part of Impulsivity-Sensation Seeking trait in the same model, and there is an item related to Impulsivity. Therefore, it is plausible to assume that the apparent inconsistency is caused by the combination of separate personality factors.
In this study, we measured Sociability that is a personality dimension of the Zuckerman‟s Alternative Five Factor model (Zuckerman, 2002). The five factors are Impulsive Unsocialized Sensation Seeking, Aggression-Hostility, Activity, Sociability, and Neuroticism-Anxiety. Zuckerman emphasized that a basic personality trait should have biological basis. Therefore, during the development of the Alternative Five model of personality, researchers factor analyzed personality scales had been used in psychobiological studies previously (Zuckerman, Kuhlman, and Camac, 1988). In a debate about the criteria of the basic personality factors in the early nineties, Zuckerman emphasized the importance of four standards. The main point of this debate was centered around the issue whether three or five factors could describe better the structure of personality. The four criteria that Zuckerman assumed were: reliability of the dimensions across ages, genders, cultures and methods; at least a moderate heritability; identification of similar kind of behavioral elements in non-human species; and association with a biological trait marker (Zuckerman, 1992). In this way, the Zuckerman-Kuhlman Personality Questionnaire (ZKPQ III-R) is based on studies connecting biological approach with taxonomic studies (Zuckerman, Kuhlman, and Camac, 1988). Sociability includes the number of friends, the amount of time spent with them, outgoingness, the preference for social activities, and the intolerance for social isolation (Zuckerman, 2002). Therefore, we assume that a more sociable person has a more complex social life that requires more developed social cognition and that provides the opportunity to build on high-level social skills.
Personality can be studied at many levels, from the genetic base to the measurement of personality traits through biochemical, neurological, psychophysiological determinants, brain structure and social behavior (Zuckerman, 1995). The neural architecture provides the final common pathway through which culture, social factors, and genetics all operate together (Davidson, 2001). Improvement in neuroimaging (mainly magnetic resonance imaging) has made quantitative analysis of brain morphology more viable. However, the majority of the studies focus on the relationships between brain structure and several psychopathologies (Matsui et al., 2000) rather than on the personality of healthy individuals. Studying inter-individual variability could provide valuable information about the neural basis of human behavior and cognition (Kanai and Rees, 2011).
As far as we know, Zuckerman‟s Alternative Five Personality traits, such as Sociability, have not been examined yet in relation to structural brain measures. The few related studies used questionnaires (NEO-PI-R, NEO-FFI) assessing the Five Factor Model of Costa and McCrae (Knutson et al., 2001; Omura, Constable, and Canli, 2005; Wright et al., 2006), Minnesota Multiphasic Personality Inventory (Matsui et al., 2000; 2002), Cloninger‟s Temperament and Character Inventory (Kaasinen et al., 2005), and Zuckerman‟s Sensation Seeking Scale (Martin et al., 2007). Regarding Extraversion, results seem to be inconsistent as we mentioned it before.
The aim of this pilot study is to examine whether the individual differences in human brain volumes are connected with the Sociability personality dimension that is associated with individuals’ social lives including their social skills. This question is highly relevant according to the framework of Evolutionary Cognitive Neuroscience (Krill et al., 2007). We intended to test in our study whether the social brain theory can be extended to the field of individual differences. This theory is supported by plenty of research carried out on non-human species, and these studies use a whole cerebrum or neocortex approach. This way, we hypothesize that the level of sociability is positively related to the volumes of the cerebrum, the neocortex, the frontal and the temporal lobes.
Materials and Methods
Participants
Twenty-five adults recruited by newspaper advertisement participated in this study. The research protocol was approved by the Ethical Committee of the Institute of Behavioural Sciences, Semmelweis University, Budapest. There were seven women and 18 men in our sample. According to a semi-structured interview, participants did not report history of any chronic or current acute illness. Subjects were free of any current drug effects including contraceptives. All subjects were Caucasians and native Hungarian speakers. The mean age was 33 years ( SD = 10.46, range = 19-55). There were two left-handed men in the study. All subjects gave informed consent before participation in the study. Two of the participants did not give their height.
Measures
Personality Questionnaire. Personality was assessed by the Hungarian version of the Zuckerman-Kuhlman Personality Questionnaire form III (Zuckerman et al., 2002), which was developed to measure the dimensions of the Alternative Five. ZKPQ III-R is a 99-item self-report questionnaire, where the subjects must decide if the statement is true or false according to their personalities. It consists of five scales (Impulsive Sensation Seeking, Aggression-Hostility, Activity, Sociability, and Neuroticism-Anxiety) and a social desirability scale (Infrequency).
The Sociability scale contains 17 items regarding to the number of friends (“I do not need a large number of casual friends”), the amount of time spent with them (“I spend as much time with my friends as I can”), how the person feels at parties (“I often find myself being the life of the party”), preference and tolerance for being alone (“Generally, I like to be alone so I can do things I want to do without social distractions”) etc.
The reliability of the Sociability scale is appropriate. The Cronbach α value revealing internal consistency was .75 in the original American sample (Zuckerman et al., 1993), but similar values were obtained in Spanish (Aluja, García, and García, 2004), German (Ostendorf and Angleitner, 1994), Chinese (Wu et al., 2000), and Japanese (Shiomi et al., 1995) samples. In a Hungarian sample (251 subjects) the Cronbach α was .78 (Rózsa and Nagy, 1998). The validity of the scale according to the peer-self agreement is good. Angleitner, Riemann and Spinath (2004) had friends or relatives of the subjects filling out the ZKPQ III-R but according to the subjects‟ personalities. The self-observer correlation was significant. In the same study, the authors investigated monozygotic and dizygotic twin pairs revealing a heritability index of .51 regarding the Sociability scale.
MRI measurements and morphometric analysis. The subjects were scanned with a 1.5-T MRI device (Siemens Magnetom Symphony). During the analysis T1-weighted (differentiating fat from water), three Dimensional Magnetization Prepared Rapid Acquisition Gradient Echo (3D MP-RAGE) sequences with 1.5 mm slice thickness were collected.
Volumes of several brain structures (right and left frontal and temporal lobes, cerebrum, and neocortex) were computed with the HAMMER (Hierarchical Attribute Matching Mechanism for Elastic Registration; version 1G5J, Department of Radiology, University of Pennsylvania Health System, Shen and Davatzikos, 2003) software package. This package uses the FSL (FMRIB Software Library; Analysis Group, FMRIB, Oxford, UK., v3.2, 2004) tools for skull stripping (BET = Brain Extractor Tool – segments brain from non-brain, and models skull and scalp surfaces) and brain tissue segmentation (FAST = FMRIB’s Automated Segmentation Tool – brain segmentation into different tissue types and bias field correction). After removing the bone tissue, the remaining data are automatically classified into three classes: gray matter, white matter and cerebrospinal fluid. The automatic classification is based on the intensities as described in Goldszal and colleagues (1998).
Next, the brain images are automatically registered and warped based on the concept of attribute vectors and by using a hierarchical approximation of the similarity function. An attribute vector is a collection of geometric attributes whose goal is to uniquely characterize every single voxel in a brain image, thereby reducing ambiguity in the matching process. Hierarchical approximation is used in order to significantly reduce local minima, which typically represent poor matches. The process is guided by those parts of the anatomy that can be identified more reliably than others. Good examples are the roots of sulci and the crowns of the gyri, which can be identified much more reliably than intermediate cortical points. The segmented volume was coregistered with Jacob atlas (Montreal Neurological Institute, Created 1997/02/19, Greg Ward; Updated Dec 17, 2001 by Noor Kabani; last revision 2003) with an elastic transformation method, and different brain structures are identified on individual studies. At last step, we visually verified the results.
We measured the volumes of the cerebrum, the neocortex, the left and the right frontal and temporal lobes. The cerebrum was defined as the white and grey matter of the brain excluding the cerebellum, the brain stem, and the thalamus-hypothalamus, while the neocortex is the grey matter part of the cerebrum except basal ganglia, hippocampal formation, and the cingulate gyrus. The frontal lobe is the brain area between the central sulcus and the lateral sulcus, while the temporal lobe extends to the lateral sulcus superiorly and to the artificially lengthened parietooccipital sulcus posteriorly. The temporal lobe includes the amygdala and the hippocampal formation.
Statistical analysis
To examine the relationship between the volumes of the frontal and temporal lobes, the neocortex, as well as the cerebrum we computed partial correlation coefficients. To exclude the effects of the age and body size differences – including sex – we used a control for age and body height (measured in cm). The fact that brain size decreases with age is well-known (Dekaban and Sadowsky, 1978; Witelson, Beresh, and Kigar, 2006), while the relationship between body height and brain size is more controversial (Spann and Dustmann, 1965; Witelson, Beresh, and Kigar, 2006). We expected positive correlations between the above areas and the Sociability score. To control of the risk of Type I. statistical error we applied the Benjamini-Hochberg correction (Benjamini and Hochberg, 1995). The correction was made in Microsoft Office ® Excel ® 2007 (© 2006 Microsoft Corporation) using an implementation described by Thissen, Steinberg, and Kuang (2002).
Results
In our sample the average brain volume (without the brain stem and the cerebellum) was 1127.89 cm3 (SD = 95.23 cm3). The mean of the Sociability score was 7.32 (SD = 3.13). Males and females differed significantly in their brain size and in the volumes of their lobes (p < .01), as well as in the volume of the neocortex (p = .014). There were no gender differences in age. The correlations between the brain regions and height and age are shown in Table 1. All of our variables follow normal distribution according to the Kolmogorov-Smirnov test.
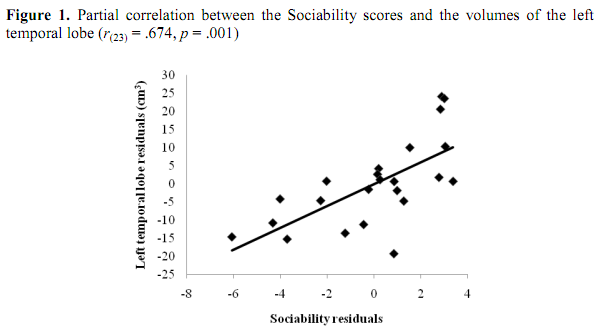
Sociability score correlated positively both with the whole and the regional brain volumes if we controlled the effects of age and body height. In details, positive correlations were observed between Sociability score and the right frontal lobe (r(23) = .475, p = .03), the left frontal lobe (r(23) = .474, p = .03), the right temporal lobe (r(23) = .647, p = .002) and the left temporal lobe (r(23) = .674, p = .001). Furthermore, we found a positive correlation between Sociability score and the volume of the cerebrum (r(23) = .55, p = .01) and the neocortex (r(23) = .663, p = .001). According to the Benjamini-Hochberg procedure, the probabilities for the correlations of the left and the right frontal lobe volumes with the Sociability score have not reached the critical &alpha-value. However, the correlation coefficients characterizing the covariations of the volumes of t he temporal lobes, the cerebrum, and the neocortex with Sociability remained significant after the corrections for multiple testing. The results are shown in Table 2.
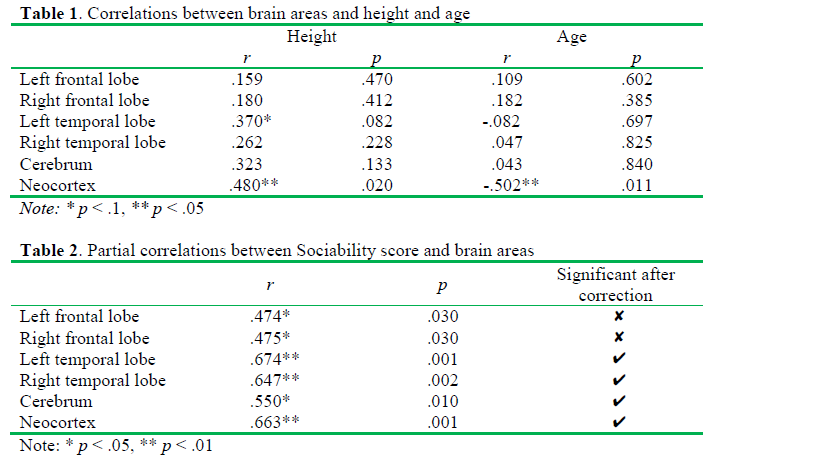
The partial correlations of Sociability with the left and the right temporal lobes are plotted on Figure 1 and Figure 2, respectively. Age and height adjusted values (residuals) of the left and right temporal lobes and Sociability score in the scatterplots were obtained via regression analyses. If we analyzed males and females separately, significant correlations between ZKPQ III-R Sociability and brain anatomy were observed in men only. Sociability correlated positively with the left (r(16) = .678, p = .008) and the right temporal lobes (r(16) = .636, p = .014), as well as the neocortex (r(16) = .634, p = .015). However, these correlations were not significant after the Benjamini-Hochberg correction. No significant correlations between Sociability and brain volumes were found in women (Table 3). Moreover, no ZKPQ III-R scales other than Sociability correlated significantly with the volumes of the above brain structures.

Discussion
In our preliminary study we examined the relationship between Sociability personality dimension and the volumes of the frontal, the temporal lobes, as well as the whole cerebrum and the neocortex. Our results supported our hypothesis that the level of Sociability is positively related to the volumes of these areas. The most remarkable correlations were found with the temporal lobes and the neocortex. These correlations were significant only in men if we analyzed females and males separately. No significant correlations were observed regarding women. Because there were only seven women who participated in this study, our result are more valid for men. These findings may have two major implications, one for evolutionary psychology and one for personality psychology; however, these suggestions have to be treated cautiously as a consequence of the small sample size.
Our results suggest that the “social brain theory” (Barton and Dunbar, 1997; Byrne and Whiten, 1988; Dunbar, 1998), which provides a possible explanation to the interspecies differences in brain size, can be extended to human interindividual differences. Furthermore, the theory is strongly supported by our observations, as brain size was related to a marker of social life among humans. However, it is not clear which factor of social life or social complexity causes this relationship, and our study cannot answer this question either, because the examined Sociability dimension is also a general concept. Many authors (Barrett, Henzi, and Dunbar, 2003; Dunbar, 1998; Dunbar and Shultz, 2007b; Spink and Cole, 2007; Stiller and Dunbar, 2007) claim that sociocognitive abilities which are necessary to live in a group are behind this relationship in animals. It is highly probable that this is applicable to humans too; a person who is more sociable has to maintain more relationships and they are better in these abilities. The findings of this study are consistent with the unpublished results cited by Dunbar (2009), that the temporal and frontal lobes are associated with theory of mind processes.
The above findings may suggest that the Sociability personality trait is characterized by more obvious neurological correlates than the Extraversion, and the inconsistent results of the studies focusing on the latter could be a consequence of this aspect. The broader interpretation of Extraversion may be a possible explanation for this fact (Zuckerman, 1991). The narrowest sense of Extraversion is Sociability. The broad Extraversion concept contains subtraits such as activity, sensation seeking, positive emotion, warmth, assertiveness. Zuckerman (1991) suggests that “the psychobiological correlates may be limited to one or another of the subtraits and not to the others” (pp. 122). The stronger correlations of Sociability with brain areas may underpin this idea; however, further research is needed to confirm our findings in other samples with a larger number of cases. The results also implicate that Sociability satisfy better the criteria of basic personality factors due to its association with a biological trait marker (Zuckerman, 1992).
In this study, we used a whole lobe or brain approach instead of a more specified analysis. First of all, our aim was to test whether the social brain theory is applicable to human individual differences. The studies supporting this theory followed a whole brain or neocortex approach. Secondly, regarding humans, theory of mind was shown to be connected with around nine different brain areas in the frontal and temporal lobes. Moreover, inconsistent results of the studies examining the relationship between Extraversion and brain structures hinder any intentions aiming to formulate specific hypotheses regarding localized brain regions. Furthermore, the examination of specific structures would increase the number of variables and thus the risk of the Type I statistical error.
Regarding the limitations of our study, we should note that the sample size was not large enough to draw definite conclusions, and this is especially true for the analysis regarding females. Furthermore, the results do not explain the underlying mechanisms of social cognition. Dunbar (2009) suggests that the combination of neurobiological and comparative approaches may provide a clarification. Moreover, we do not know due to the limitations of a correlation study if larger brains cause higher Sociability or higher Sociability causes larger brains. It is also possible that a third variable is behind these correlations. Furthermore, studies using questionnaires could have some inherent limitations too. The questionnaires cannot measure the psychological variables directly, and respondents may answer the questions superficially or they may intend to satisfy social desirability. Behavioral observations would be ideal to confirm our findings.
To summarize we would like to emphasize that only tentative conclusions can be drawn from these results. Indeed, these findings have considerable implications for two major fields of psychology.
Acknowledgements: This research was supported by the by the National Office for Research and Technology (NKFP-1B/020/04).
Received 10 Dec 2010; Revision submitted 29 April 2011; Accepted 01 May 2011
References
Abu-Akel, A. (2003). A neurobiological mapping of theory of mind. Brain Research Reviews, 43, 29-40.
Aluja, A., García, Ó., and García, L. F. (2004). Replicability of the three, four, and five Zuckerman‟s personality super-factors: exploratory and confirmatory factor analysis of the EPQ-RS, ZKPQ and NEO-PI-R. Personality and Individual Differences, 36, 1093-1108.
Angleitner, A., Riemann, R., and Spinath, F. M. (2004). Investigating the ZKPQ-III-R: psychometric properties, relations to the five-factor model, and genetic and environmental influences on its scales and facets. In Stelmack, R. M. (Ed.), On the Psychobiology of Pers onality: Essays in Honor of Marvin Zuckerman (pp. 89-105). Oxford: Elsevier.
Barrett, L., Henzi, P., and Dunbar, R. (2003). Primate cognition: from „what now?‟ to „what if?‟. Trends in Cognitive Sciences, 7, 494-497.
Barton, R., and Dunbar, R. I. M. (1997). Evolution of the social brain. In Whiten, A., and Byrne, R. W. (Eds.), Machiavellian intelligence II (pp. 240-263.) Cambridge, UK: Cambridge University Press.
Benjamini, Y., and Hochberg, Y. (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society, Series B, 57, 289-300.
Byrne, R. W., and Corp, N. (2004). Neocortex size predicts deception rate in primates. Proceedings of the Royal Society B-Biological Sciences, 271, 1693-1699.
Byrne, R. W., and Whiten, A. (1988). Machiavellian intelligence. Oxford, UK: Oxford University Press.
Costa, P. T., and McCrae, R. R. (1992). The revised NEO Personality Inventory (NEO-PI-R) professional manual. Odessa, FL: Psychological Assessment Resources.
Costa, P. T., McCrae, R. R., and Dye, D. (1991). Facet scales for Agreeableness and Conscientiousness: a revision of the NEO Personality Inventory. Personality and Individual Differences, 12, 887-898.
Davidson, R. J. (2001). Toward a biology of personality and emotion. Annals of the New York Academy of Sciences, 935, 191–207.
Dekaban, S., and Sadowsky, D. (1978). Changes in brain weights during the span of human life: Relation of brain weights to body heights and body weights. Annals of Neurology, 4, 345-356.
Dunbar, R. I. M. (1992). Neocortex size as a constraint on group-size in primates. Journal of Human Evolution, 22, 469-493.
Dunbar, R. I. M. (1998). The social brain hypothesis. Evolutionary Anthropology, 6, 178-190.
Dunbar, R. I. M. (2009). Darwin and the ghost of Phineas Gage: Neuro-evolution and the social brain. Cortex, 45, 1119-1125.
Dunbar, R. I. M., McAdam, M. R., and O‟Connell, S. (2005). Mental rehearsal in great apes (Pan troglodytes and Pongo pygmaeus) and children. Behavioral Processes, 69, 323-330.
Dunbar, R. I. M., and Shultz, S. (2007a). Understanding primate brain evolution. Philosophical Transactions of the Royal Society of London, 362B, 649-658.
Dunbar, R. I. M., and Shultz, S. (2007b). Evolution in the social brain. Science, 268, 1578-1584.
Gallagher, H. L., and Frith, C. D. (2003). Functional imaging of „theory of mind‟. Trends in Cognitive Sciences, 7, 77-83.
Goldszal, A. F., Davatzikos, C, Pham, D. L., Yan, M. X. H., Bryan, R. N., and Resnick, S. M. (1998). An image processing system for qualitative and quantitative volumetric analysis of brain images. Journal of Computer Assisted Tomography, 22, 827-837.
Kaasinen, V., Maguire, R. P., Kurki, T., Brück, A., and Rinne, J. O. (2005). Mapping brain structure and personality in late adulthood. Neuroimage, 24, 315-322.
Kanai, R., and Rees, G. (2011). The structural basis of inter-individual differences in human behaviour and cognition. Nature Reviews Neuroscience, 12, 231-242.
Knutson, B., Momenan, R., Rawlings, R. R., Fong, G. W., and Hommer, D. (2001). Negative association of neuroticism with brain volume ratio in healthy humans. Biological Psychiatry, 50, 685-690.
Krill, A. L., Platek, S. M., Goetz, A. T., and Shackelford, T. K. (2007). Where Evolutionary Psychology meets Cognitive Neuroscience: A précis to Evolutionary Cognitive Neuroscience. Evolutionary Psychology, 5, 232-256.
Kudo, H., and Dunbar, R. I. M. (2001). Neocortex size and social network size in primates. Animal Behavior, 62, 711-722.
Lewis, K. (2001). A comparative study of primate play behavior: Implications for the study of cognition. Folia Primatologica, 71, 417-421.
Lindenfors, P. (2005). Neocortex evolution in primates: the „social brain‟ is for females. Biological Letters, 1, 407.
Martin, S. B., Covell, D. J., Joseph, J. E., Chebrolu, H., Smith, C. D., Kelly, T. H., Jiang, Y., and Gold, B. T. (2007). Human experience seeking correlates with hippocampus volume: Convergent evidence from manual tracing and voxel-based morphometry. Neuropsychologia, 45, 2874-2881.
Matsui, M., Ruben, C. G., Turetsky, B. I., Yan, M. X. H., and Gur, R. E. (2000). The relation between tendency for psychopathology and reduced frontal brain volume in healthy people. Neuropsychiatry, Neuropsychology, and Behavioral Neurology, 13, 155-162.
Matsui, M., Yoneyama, E., Sumiyoshi, T., Noguchi, K., Nohara, S., Suzuki, M., Kawasaki, Y., Seto, H., and Kurachi, M. (2002). Lack of self-control as assessed by a personality inventory is related to reduced volume of supplementary motor area. Psychiatry Research-Neuroimaging, 116, 53-61.
Omura, K., Constable, R.T., and Canli, T. (2005). Amygdala gray matter concentration is associated with extraversion and neuroticism. Cognitive Neuroscience and Neuropsychology, 16, 1905-1908.
Ostendorf, F., and Angleitner, A. (1994). A comparison of different instruments proposed to measure the Big Five. European Review of Applied Psychology, 44, 45-53.
Pawlowski, B. P., Lowen, C. B., and Dunbar, R. I. M. (1998). Neocortex size, social skills and mating success in primates. Behaviour, 135, 357-368.
Powell, J., Lewis, P., Dunbar, R., García-Fiñana, M., and Roberts, N. (2010). Orbital prefrontal cortex volume correlates with social cognitive competence. Neuropsychologia, 48, 3554-3562.
Reader, S. M., and Laland, K. N. (2002). Social intelligence, innovation, and enhanced brain size in primates. Proceedings of the National Academic Sciences of the United States of America, 99, 4436-4441.
Rózsa, S., and Nagy, J. (1998). Ötfaktoros kérdőívek hazai adaptációjának újabb eredményei. XIII. Országos Pszichológiai Tudományos Nagygyűlés, Előadáskivonatok, 268. Shen, D., and Davatzikos, C. (2003). Very high-resolution morphometry mass-preserving deformations and HAMMER elastic registration. Neuroimage, 18, 28-41.
Shultz, S., and Dunbar, R. I. M. (2007). The evolution of the social brain: anthropoid primates contrast with other vertebrates. Proceedings of the Royal Society B, 274, 2429-2436.
Shiomi, K., Shigemori, Y., Kuhlman, D. M., Joireman, J. A., Sato, M. (1995). Constructing and evaluating a Japanese version of the Zuckerman-Kuhlman Personality Questionnaire. Hyogo University of Teacher Education Journal, 15, 1-12.
Spann, W., and Dustmann, H. O. (1965). Das menschliche Hirngewicht und seine Abhängigkeit von Lebensalter, Kö rperlänge, Todesursache und Beruf. Deutsche Zeitschrift für die Gesamte Gerichtliche Medizin, 566, 299-317.
Spink, A., and Cole, C. (2007). Information behavior: A socio-cognitive ability. Evolutionary Psychology, 5, 257-274.
Stiller, J., and Dunbar, R. I. M. (2007). Perspective-taking and memory capacity predict social network size. Social Networks, 29, 93-104.
Thissen, D., Steinberg, L., and Kuang, D. (2002). Quick and easy implementation of the Benjamini-Hochberg procedure for controlling the false positive rate in multiple comparisons. Journal of Educational and Behavioral Statistics, 27, 77-83.
Witelson, S. F., Beresh, H., and Kigar, D. L. (2006). Intelligence and brain size in 100 postmortem brains: Sex, lateralization and age factors. Brain, 129, 386-398.
Wright, C. I., Williams, D., Feczko, E., Barett, L. F., Dickerson, B. C., Schwartz, C. E., and Wedig, M. M. (2006). Neuroanatomical correlates of extraversion and neuroticism. Cerebral Cortex, 16, 1809-1819.
Wu, Y. X., Wang, W., Du, W. Y., Li, J., Jiang, X. F., and Wang, Y. H. (2000). Development of a Chinese version of the Zuckerman-Kuhlman Personality Questionnaire: reliabilities and gender/age effects. Social Behavior and Personality, 28, 241-250.
Zuckerman, M. (1991). The psychobiology of personality. New York: Cambridge University Press.
Zuckerman, M. (1992). What is a basic factor and which factors are basic? Turtles all the way down. Personality and Individual Differences, 13, 675-681.
Zuckerman, M. (1995). Good and bad humors. Biological bases of personality and its disorders. Psychological Science, 6, 325-332.
Zuckerman, M. (2002). Zuckerman-Kuhlman Personality Questionnaire (ZKPQ). An alternative five-factor model. In De Raad, B and Perugini, M. (Eds.), Big Five Assessment (pp 377-396). Gottingen: Hogrefe and Huber Publishers.
Zuckerman, M., Kuhlman, D. M., and Camac, C. (1988). What lies beyond E and N? Factor analyses of scales believed to measure basic dimensions of personality. Journal of Personality and Social Psychology, 54, 96-107.
Zuckerman, M., Kuhlman, D. M., Joireman, J., Teta, P., and Kraft, M. (1993). A comparison of three structural models for personality: The Big Three, the Big Five,and the Alternative Five. Journal of Personality and Social Psychology, 65, 757-768.