|
National Institute of Psychiatry and Neurology, *Epilepsy Center, °Department of Radiology, Budapest Levelezô szerzô/Correspondence: Dr. Halász Péter, Országos Pszichiátriai és Neurológiai Intézet, Epilepszia Centrum, H-1021 Budapest, Hûvösvölgyi út 116. Telefon: (1) 391-5436, fax: (1) 391-5438, e-mail: halasz@opni.hu Közlésre érkezett: 2003. június 24. Elfogadva: 2003. július 3 ABSTRACTWe present a patient in whom retrosplenial tumour was associated with epileptic symptoms characterized by complex partial seizures and widespread interictal and ictal epileptiform EEG abnormalities The patient had verbal memory deficit symptoms as well. After surgical removal of the tumour (oligoastrocytome) the clinical symptoms and EEG signs disappeared. The characteristics of our patient demonstrate the possible role of the retrosplenial area in widespread epileptic symptoms and in the regulation of secondary bilateral synchrony, in addition to its recently described importance in the memory functions. Keywords: retrosplenial cortex, tumour, secondary epileptogenesis, secondary bilateral synchrony, memory network ABSZTRAKTA retrosplenialis régióban keletkezô epilepszia tünetei kevéssé ismertek. Egy beteg esetét ismertetjük, akinek az oligoastrocytomája a retrosplenialis régióban helyezkedett el és epilepsziás tünetekkel szövôdött. Mind a rohamok, mind az interictalis és ictalis EEG-tünetek szerteágazó, kétoldali, részben halántéklebenyi, részben hypothalamicus rohamgenezisre, illetve terjedésre utaltak. Az epilepsziás tüneteken kívül verbális memóriadeficitet észleltünk. A tumor sebészeti eltávolítása után mind a rohamok, mind az EEG-tünetek megszûntek. A sajátosan távoli epilepsziás elektromos jelenségek arra utalhatnak, hogy a retrosplenialis régió – a memóriafunkciókban az utóbbi idôben leírt szerepén túlmenôen – szerepet játszhat a másodlagos bilaterális szinkronizáció mechanizmusában. Kulcsszavak: retrosplenialis kéreg, daganat, másodlagos epileptogenesis
IntroductionHitherto the retrosplenial area was not known to be a locus where lesions induce a characteristic epilepsy syndrome. Recent neuropsychological and neuroimaging studies revealed that the retrosplenial cortex probably play an important role in memory, especially in navigation processes and recognition of familiarity and it is supposed to have a role in connecting emotions with memories1–3. It is well-known that this region is abundantly interconnected with the hippocampal formation and the hypothalamus4, 5. Patients with splenial tumours had memory dysfunctions and visual perceptual disturbances6, 7. Also the retrosplenial cingular region is among the areas described by Tükel and Jasper8, and Penfield and Jasper9, where epileptogenic lesions are frequently associated with the EEG phenomena known as “secondary bilateral synchrony” (SBS). We present a patient in whom the retrosplenial tumour was associated with different seizure types and widespread interictal and ictal EEG discharges. Case report
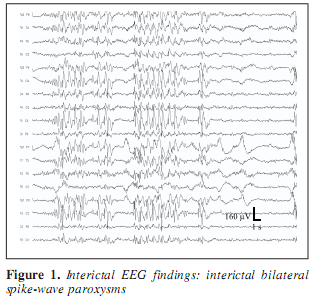
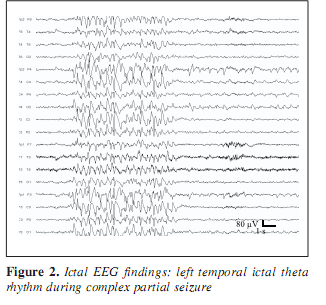
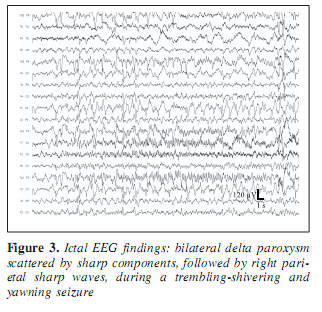
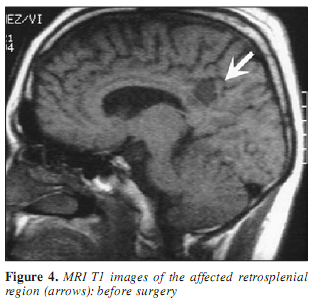
A 30-year-old man was known to have two types of epileptic seizures from age 18: rare generalized tonic-clonic seizures, and fits characterized by loss of speech and contact, followed by amnesia occuring monthly. Seizures did not respond to the available antiepileptic drugs including phenytoin, carbamazepin, valproate, vigabatrin, clobazam, and lamotrigin. At age 19, the EEG showed left temporal spiking during NREM sleep. Occasionally episodic bilateral synchronous spike-wave paroxysms appeared in the interictal EEG (Figure 1) and right central sharp wave series were also present. Due to drug resistency, presurgical evaluation was performed including long-term video-EEG monitoring. During video-monitoring, after partial withdrawal of his antiepileptic drugs (vigabatrin withdrawal with maintenance of carbamazepine) several epileptic seizures were observed with variable features. During three days of monitoring two times left (contralateral) temporal ictal rhythmic theta activity was detected while he was nonresponsive (Figure 2). Frequent clusters of bilateral high amplitude delta paroxysms appeared several times scattered with sharp waves. During these epochs he did not respond and showed peculiar trembling-shivering bilateral movements involving both arms preceded or conjoined with yawning. After several paroxysms he showed nominal aphasia and also difficulties in recognition pictures, compensated by apprehension and blasphemy. Several times the bilateral EEG paroxysms were followed by focal sharp waves over the right parietal region (Figure 3). MRI revealed right sided retrosplenial small T1 hyposignal area (Figure 4).
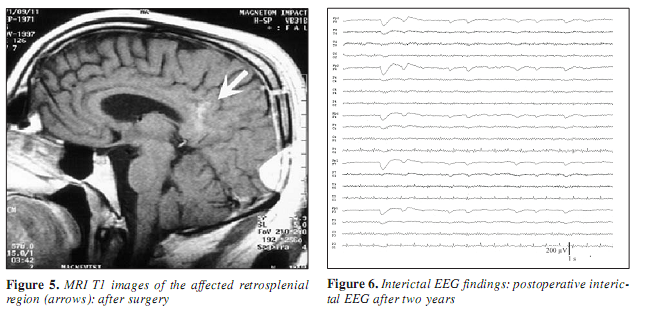
On the basis of the presurgical evaluation, a lesionectomy was performed in October 1997. Histological examination of the resected tissue confirmed alien tissue identified as oligoastrocytoma. For five years he has been seizure free on carbamazepine monotherapy, except one tonic-clonic generalised seizure, when, after one postoperative year, medication was reduced. Interictal EEG abnormalities completely disappeared after the surgery (Figure 5, 6). Neuropsychological examination revealed normal intellectual abilities (Wechsler Adult Intelligence Scale IQ=99), but immediate and delayed story recall showed deficits in logical memory. Single words were remembered well. Hence the retrosplenial area is assumed to play role in emotional learning, a memory task comparing learning of emotionally charged and neutral words was performed. The higher rank of emotional learning did not seem to be harmed in our case. Hi Control neuropsychological tests three years after the surgery showed identical results compared with the presurgical findings.
DiscussionTwo types of seizures were registered, at least from the point of view the ictal EEG symptoms. The ictal EEG showed in two times left temporal seizure pattern with characteristic rhythmic ictal theta activity on the contralateral side to the epileptogenic lesion. During the contralateral temporal seizures, only a brief loss of contact could be observed. The earlier registered left temporal spike focus in sleep seems to be consonant with this kind of seizure mechanism. The other, more frequent type of seizures which were observed in clusters, at least during the video-monitoring, were peculiar brief fits with trembling-shivering conjoined by yawning and loss of contact. During this kind of fits the EEG showed bilateral delta paroxysms scattered occasionally with sharp wave elements without consequent focality. Duration of the paroxysms was from three to seven seconds. On the basis of the shivering-trembling and yawning symptomatology and the bilateral slow wave paroxysms on the EEG the possibility of a hypothalamic seizure spread can be raised. Both kinds of seizures originated from the retrosplenial lesion since they disappeared after surgery. The presumed local retrosplenial seizure discharge was not projected to the convexity scalp EEG during the seizures, probably due to the hidden localisation. However, after seizures a train of homolateral local parasagittal PLED like discharge appeared as a kind of afterdischarge. The two types of seizures had totally different EEG symptomatology, so they can be interpreted the activity of two different seizure networks with the same origin. Based on clinical and EEG symptomatology one of them probably involves the temporal and the other the hypothalamic network. This would imply that the symptomatogenic region of the seizures was remote to the retrosplenial site. The abundant connections of the retrosplenial area with the hippocampus and hypothalamus4, 5 provide a solid anatomical basis for this. Recent functional neuroimaging studies have shown an important role of the retrosplenial cortex (Brodman areas: 23, 30, 31) in memory functions especially in the recognition of familar faces and in navigation as well as in the emotional significance of memories1, 3, 10. These findings suggest that the retrosplenial cortex should be one of the structures in a network responsible for memory functions requiring synchronised actions of different areas in the two hemispheres. The deficits in logical memory performance in our patient support the role of the retrosplenial cortex in memory functions. Concerning the mechanism of contralateral temporal spiking and contralateral temporal seizure activity, we hypothetise that the retrosplenial epileptogenic region may emanate discharges either to the homolateral or to the contraleral side. The pathway through which the contraleral spread occured is probably the posterior hippocampal comissure11, and this structure is in close vicinity of the retrosplenial area. Secondary bilateral synchrony also disappeared after the surgical intervention, supporting the role of the retrosplenial area also in the regulation of bilateral epileptiform activity. As it was shown already in the early work of Tükel and Jasper8, and also later by Williamson12, the medial surface of the parietal lobe might be a part of the network playing a key role in this phenomenon. REFERENCES1. Maguire EA. The retrosplenial contribution to human navigation: a review of lesion and neuroimaging findings. Scand J Psychol 2001;42(3):225-38. 2. Shah NJ, Marshall JC, Zafiris O, el al. The neural correlates of person familiarity. A functional magnetic resonance imaging study with clinical implications. Brain 2001;124:804-15. 3. Maddock EA. The retrosplenial cortex and emotion: new insights from functional neuroimaging of the human brain. Trends Neurosci 1999;22(7):310-6. 4. Wyss JM, Van Groen T. Connections between the retrosplenial cortex and the hippocampal formation in the rat. Hippocampus 1992;2(1):1-11. 5. Maguire EA, Vargha-Khadem F, Mishkin M. The effects of bilateral hippocampal damage on fMRI regional activations and interactions during memory retrieval. Brain 2001;124:1156-70. 6. Rudge P, Warrington EK. Selective impairment of memory and visual perception in splenial tumours. Brain 1991;114:349-60. 7. Iwasaki S, Arihara T, Torii H, et al. A case of splenial astrocytoma with various neuropsychological symptoms. No To Shinkei 1993;45:1067-73. 8. Tükel K, Jasper HH. The EEG in parasagittal lesions. Electroenceph Clin Neurophysiol 1952;4:481. 9. Penfield W, Jasper H. Epilepsy and the functional anatomy of the human brain. Boston: Little, Brown & Co.; 1954. p.896. 10. Vogt BA, Absher JR, Bush G. Human retrosplenial cortex: where is it and is it involved in emotion? Trends Neurosci 2000;23(5):195-7. 11. Kobayashi Y, Amaral DG. Macaque monkey retrosplenial cortex: I. three-dimensional and cytoarchitectonic. J Comp Neurol 2000;426:339-65 12. Williamson PD, Boon PA, Thadani VM, et al. Parietal lobe epilepsy: diagnostic considerations and results of surgery. Ann Neurol 1992;31:193-201. |