|
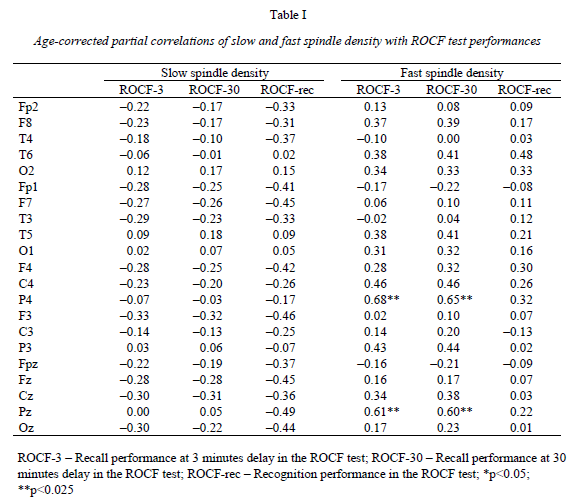
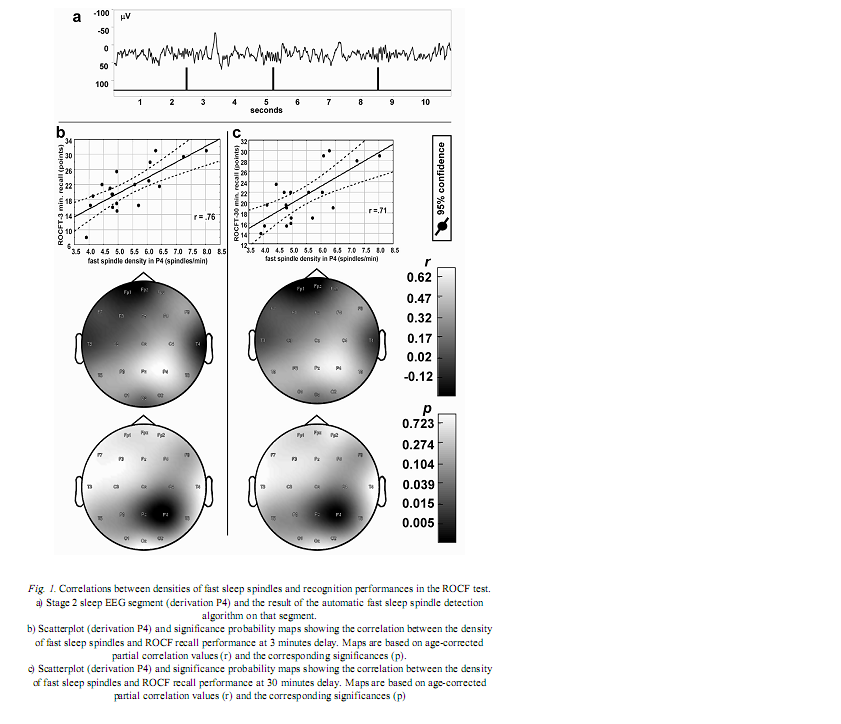
Institute of Behavioural Sciences, Semmelweis University, Nagyvárad tér 4, H-1089 Budapest, Hungary Received: June 5, 2007 Accepted: December 14, 2007 AbstractIndividual differences in human sleep EEG spindling were shown to be associated with psychometric measures of cognitive ability. Previous results revealed a frequency- and region specificity of this effect, suggesting that only fast, but not slow spindle-related oscillatory activity over the frontal region correlated with cognitive performance. Our aim is to test the hypothesis that region-specific spindletype oscillatory activity is related to specific cognitive abilities reflecting the cortical localization of the corresponding cognitive function. The visuospatial abilities are the focus of the present report. Nineteen healthy volunteers were tested with the Rey-Osterrieth Complex Figure (ROCF) test and memory performances correlated with the spindle analysis of the second night’s polysomnographic recordings. Correlations were age-corrected and subjected to descriptive data analysis. ROCF recall performances at 3 and 30 minutes delay, correlated positively and significantly with fast sleep spindle density measured over the right parietal area. No significant relationship between recognition performance and sleep EEG variables emerged. Slow spindle density did not correlate with test performances. Our findings converge with other data suggesting the involvement of right parietal functioning in visuospatial abilities. Moreover, these results support the hypothesis that region-specific differences in fast sleep spindling could be markers of specific neuropsychological performances. Keywords: cortical synchronization, electroencephalography, neuropsychological tests, parietal lobe, laterality, sleep stages IntroductionSleep spindles are commonly known as groups of rhythmic waves of ~12–16 Hz characterized by progressively increasing, then gradually decreasing amplitudes, being the hallmarks of human stage 2 sleep electroencephalogram (EEG), but appearing also during deeper stages of NREM sleep (10). According to neurophysiological studies, spindle oscillations reflect the synchronized rhythmic hyperpolarization-rebound sequences of thalamocortical cells. GABAergic thalamic reticular neurons are suggested pacemakers of spindle oscillations, but there is data supporting the concept that phases of cortical depolarization during the slow oscillation drive the thalamocortical spindle activity (43, 44). There is evidence for the idea that monosynaptic and polysynaptic horizontal collaterals of layer V neurons can play a significant role in the initiation of spindles (47). Thus, cortical connectivity is related to these forms of sleep EEG oscillatory activity. As a consequence sleep EEG oscillations could reflect cognitive functions or abilities based on cortical connectivity. Indeed recent evidences suggest the relationship of certain sleep EEG oscillations with cognitive functioning. Fast frequency sleep spindling measured over the frontal region was shown to correlate positively with general cognitive ability (6). Similar relationships between sleep spindling and cognition were unravelled with regard to general cognitive ability (40) as well as perceptual/analytical skills and performance IQ (21). Positive correlations between sleep spindles and overnight verbal or visuospatial memory improvement (8, 39) or verbal learning performance (23) were also reported. Moreover, the relationships between sleep spindles and overnight verbal or visuospatial memory improvement were characterized by a region-specificity: left prefrontal spindling accounted for the differences in verbal while right parietal spindling for those in visuospatial memory improvement (8, 9). Other studies reported associations between parahippocampal electrocorticographic as well as temporal scalp EEG measures of the sleep slow oscillation and visuospatial memory performances (5). Beside the-well known involvement of the medial temporal lobe system in visuospatial memory performance (38, 42), the significance of extrahippocampal neocortical regions including the right hemisphere (20), and especially the right parietal cortex (30) was also reported. Here we address the question of the relationship of the above mentioned sleep EEG oscillations (sleep spindles) with visuospatial memory performance. Current work is a part of a larger project aiming to reveal the relationships between individual levels of different cognitive performances and sleep-EEG characteristics. Our actual choice of analysing visuospatial memory performance was based on the reported independence of this ability from general mental ability or intelligence (22) as well as on its relatively unequivocal neuropsychological localization (20, 30). Future work is planned to detect associations between other cognitive performances and sleep EEG features. We follow the sleep-cognition hypothesis, looking for the interrelationship between individualspecific night-time sleep EEG features and daytime cognitive performance (3, 5, 15, 16, 35). According to this hypothesis it is not only the overnight memory improvement (9), but also the baseline, presleep performance what correlates with sleep EEG. Furthermore, we hypothesize that the topography of positive correlations reflects the involvement of the right parietal cortex in visual memory (30, 34). We consider this distinction a highly important one, as the sleep-cognition hypothesis suggests that individual differences between subjects at the very beginning of the learning process are reflected in their sleep EEG activity, while the memory consolidation theories of sleep emphasize the differences in the process of post-learning consolidation. It is important to note that in humans two types of sleep spindles have been described: slow spindles with a frequency of about 12 Hz and predominant frontal localization and fast spindles of about 14 Hz with predominant, but not exclusive centroparietal localization (2, 10, 31, 49). There are large interindividual differences in the relative predominance of slow and fast spindle frequency activities (48). The physiological basis and functional significance of these two types of sleep spindles is a controversial issue (10, 28, 50, 51). Given these controversies as well as our previous result revealing an exclusive association of fast sleep spindling with general cognitive ability (6) here we perform a differentiated analysis for slow and fast sleep spindles. Subjects and MethodsOur hypothesis was tested on the sample and polysomnographic data of a previous study reporting the relationship between general cognitive ability and neural oscillations of sleep (6). The sample consisted of 19 health Subjects slept two consecutive nights in the sleep laboratory, according their preferred sleep schedules. Sleep was monitored by standard polysomnography including 21 EEG derivations of the 10–20 system (Fp1, Fp2, Fpz, F3, F4, Fz, F7, F8, C3, C4, Cz, T3, T4, T5, T6, P3, P4, Pz, O1, O2, Oz) referred to the contralateral mastoids, as well as bipolar electro-oculography, submental electromyography and electrocardiography with collodion-fixed Ag/AgCl electrodes. We used the right mastoid as a reference for the midline EEG electrodes. Signals from all electrodes were high-pass filtered at 0.33 Hz, amplified and digitized at 128 Hz with 12 bit resolution. Subjects spent the day between the two consecutive recording nights in the department completing psychometric tests at fixed time schedules. Visual memory was assessed between 9 and 10 AM by the ROCF test (29). While performing this test, the subjects had to copy a complex figure consisting of various geometrical components. The test instruction contained no hints to memorize the figure. The subjects had to redraw the Figure 3 minutes and 30 minutes later relying entirely on visual memory. Based on the 18-item (36-point) scoring system three indices of visual memory were used in this study: immediate recall (performance at 3 minutes delay), delayed recall (30 minutes delay), and recognition trial (29). The recognition trial is not available for two of the subjects, thus the statistics for this subtest is based on N=17 subjects. Outside the standard testing, subjects were restricted in doing major physical and mental effort in order to avoid large interindividual differences in daytime activity and its consecutive effects on sleep. No daytime naps were allowed and subjects were instructed to refrain from drinking alcohol or taking any medication. Sleep recordings of the second nights were visually scored according to standard criteria (37). After visual artefact rejection, 4 second epochs were Hanning-tapered, zero-padded to 16 seconds and Fast-Fourier transformed in order to get the average amplitude spectra for stage 2 sleep with 0.0625 Hz resolution. The counting of slow, and fast sleep spindles was performed by a previously validated method, based on the individual adjustment of frequency and amplitude criteria for slow and fast sleep spindles, respectively. In short the principle of sleep spindle detection is the idea that individual spindles are those groups of waves which last at least 0.5 seconds and contribute to one or two of the major peaks in the 9–16 Hz average amplitude spectra of stage 2 sleep EEG. Individual-specific spectral peaks were formalized by calculating the zero crossing points of their second order derivatives (6) (see Fig. 1a for an example of spindle detection in derivation P4). Slow and fast spindle densities of individual NREM-REM cycles were calculated by dividing the number of slow and fast spindles by minutes of stage 2 sleep during which they occurred. All-night spindle densities and spindle frequency activities were obtained by averaging the data of individual NREM-REM cycles. We correlated the sleep EEG variables with ROCF test performances with the statistical control of age. In order to obtain a better localization of regions with significant correlations between EEG and the ROCF test performances the partial correlations between ROCF test scores and sleep EEG data were represented by significance probability maps (25). As multiple testing can inflate type I error, the procedure of descriptive data analysis (1) adapted to quantified neurophysiology with mapping (14) was applied to our partial correlational (age-corrected) data. This procedure tests the global null hypothesis (“all individual null hypotheses in the respective region are true”) at level α=0.05, against the alternative that at least one of the null hypotheses is wrong. According to Abt (1) and Duffy et al. (14) local, uncorrected significances at the level of α=0.05 (descriptive significances) define the Rüger’s areas. If N is the number of electrodes in the Rüger’s area, the investigator is required to choose a minimal number of unspecified null hypotheses (M), less than N, to be nominally rejected at a new, more conservative α level. Typically the value M/N is 1/2 or 1/3. The corresponding new α levels for these values are α/2=0.025 and α/3=0.017, respectively. We will use an M/N value of 1/2 and a corresponding new α of 0.025 in our analyses. If any M values (half of the correlation coefficients if M/N=1/2) within the Rüger’s area individually reach the new α level of significance the overall null hypothesis is rejected for the Rüger’s area at the 0.05 level. This means that for at least one EEG derivation in the Rüger’s area the relationship is significant, allowing the investigator to make global confirmatory statement with controlled uncertainty. ResultsMean recall performance of the ROCF was M=21.13 (SD=5.97) for 3 minutes delay and M=21.02 (SD=4.98) for 30 minutes delay. Mean recognition performance was M=19.8 (SD=2.18). There was no significant correlation between ROCF test performance and IQ as measured by the Raven Progressive Matrices Test (r=0.06, 0.19, and 0.13 for 3 minutes delay, 30 minutes delay and recognition, respectively). The density of slow sleep spindles did not correlate significantly with ROCF test performances (Table I). However, the density of fast sleep spindles calculated for derivations P4 and Pz correlated positively with recall performance of the ROCF at 3 minutes delay (P4: r=0.68, p=0.002; Pz: r=0.61, p=0.009). The Rüger’s area defined by these descriptive significances consists of two electrodes and extends mainly over the right parietal area (Fig. 1b). As both of these correlations correspond to the new α level of 0.025 (Table I), descriptive data analysis indicates at least one significant effect in this area.
The above effect was not specific for 3 minutes delay as the density of fast sleep spindles calculated for derivations P4 and Pz correlated positively with recall performance of the ROCF at 30 minutes delay as well (P4: r=0.65, p=0.003; Pz: r=0.60, r=0.011). Consequently, the same Rüger’s area is defined for delayed (30 minutes) recall performances (Fig. 1c). Again both of the correlations correspond to the more conservative α level of 0.025 (Table I), hence descriptive data analysis indicates at least one significant effect in this area. DiscussionThere is a large interindividual and a relatively low intraindividual variance in the frequency and amplitude of slow and fast sleep spindles (48). Instead of using predefined frequency and amplitude criteria we adjusted these values individually for slow and fast spindles. Nevertheless, there is reasonable evidence for the functional Frontally derived fast sleep spindle density predicts general fluid intelligence (6). This result parallels the findings showing that intelligence-differences are related to variations in prefrontal cortical grey matter volume (46). Our results point to the fact that visuospatial memory abilities as measured by the ROCF test are predicted by fast sleep spindling in the right parietal area. Moreover, there is parallel evidence for the association between right hemisphere’s grey matter volume and visual memory performance assessed by the ROCF test (20). Based on these findings we speculate that local cortical grey matter volume is the common neuroanatomical basis for both the individual differences in the region-specific fast sleep spindle density and for variations in neuropsychological performance measured by psychological instruments. This reasoning fits well with the observation of a reduced number of sleep spindles in early stages of Creutzfeldt-Jakob disease (12), Alzheimer disease (33) and schizophrenia (17) as these syndromes are associated with cortical atrophy (4, 19, 27) and impaired visual memory (7, 26, 24). Although, there is a possibility to find a more or less parallel impairment of visuospatial memory and general mental ability in the above-mentioned clinical groups, the correlation between these two cognitive measures is usually low in healthy controls (22). We found no significant correlations between visuospatial memory abilities and general mental ability in our subjects. These findings provide further confirmation for the relative independence of visuospatial memory and general intelligence. Although suggested genetic origins of individual differences in memory functioning may contribute to our present findings (32), there is also evidence for changes in grey matter induced by training (13). The latter result suggests that training may alter the neuroanatomical bases of both sleep EEG and visual memory. There are results demonstrating that the pattern of the EEG power distribution in NREM sleep is characteristic for an individual, possibly reflecting individual traits of functional anatomy (11, 18). The high internight reliability of sleep spindle density (41) and sleep- EEG spectra (45) might point in a similar direction. Our present and previous findings (6) showing frequency- and region specific correlations of sleep spindles with visuospatial memory as well as of sleep spindles with general cognitive ability add a behavioural/cognitive dimension to the functional neuroanatomical traits reflected in sleep-EEG activities. Further examples for such associations of behavioral/cognitive and sleep-EEG measures are reported for the frontal lobe-specific neuropsychological performance related to the sleep-slow oscillation measured over the left prefrontal area (3), as well as for superior memorizing related to increased periodic arousal fluctuation measured by the cyclic alternating pattern rate during NREM sleep (16). By completing the individual fingerprint theory of sleep-EEG differences (11, 18) with the behavioural/cognitive dimension, a new possibility for unravelling the neural bases of human cognitive functions emerges. AcknowledgementsThis work was supported by the National Office for Research and Technology (NKFP-1B/020/04) and the National Research Fund (OTKA T-048927 and OTKA TS-049785). The first author is supported by the János Bolyai Research Fellowship of the Hungarian Academy of Sciences. REFERENCES1. Abt K: Descriptive data analysis: A concept between confirmatory and exploratory data analysis.
|