|
DOI: 10.1016/j.bandc.2012.01.006
Authors:
Péter Simora, Péter Pajkossya, Klára Horváthb, Róbert Bódizsb,c
aDepartment of Cognitive Science, Budapest University of Technology and Economics, Egry József u.1. Tépület./V.em., Budapest H-1111, Hungary
b Institute of Behavioural Sciences, Semmelweis University, Nagyvárad tér 4, Budapest H-1089, Hungary
c HAS-BME Cognitive Science Research Group, Hungarian Academy of Sciences, Egry József u.1. Tépület./V.em., Budapest H-1111, Hungary
Abstract
Nightmare disorder is a prevalent parasomnia characterized by vivid and highly unpleasant dream experiences during night time sleep. The neural background of disturbed dreaming was proposed to be associated with impaired prefrontal and fronto-limbic functioning during REM sleep. We hypothesized that the impaired prefrontal and fronto-limbic functioning in subjects with frequent nightmares would be reflected at the behavioral level during waking tasks as well. 35–35 Subjects with frequent nightmares and matched controls participated in Study 1, involving an Emotional Go/NoGo, an Emotional Stroop task, and a Verbal Fluency task. Nightmare subjects exhibited longer reaction times in the Emotional Go/NoGo and Emotional Stroop tasks. Moreover, they committed more perseveration errors and showed less fluent word generation in the Verbal Fluency task. Nightmare subjects showed an overall slowing irrespective of the valence of the stimuli. While the effects of sleep quality and waking anxiety were associated to these deficits in some cases, these factors could not solely explain the difference between the two groups. In Study 2, 17 subjects with frequent nightmares and 18 controls were compared by a Color-word and an Emotional, block design Stroop task in order to avoid the slow effects of emotional interference potentially caused by previous items. Nightmare subjects were characterized by an overall slowing in the Emotional Stroop task, irrespective of the valence of the stimuli. In the Color-word Stroop task, nightmare subjects were not significantly slower in comparison with controls. Our results suggest that individuals with frequent nightmares are impaired in executive tasks involving the suppression of task-irrelevant semantic representations.
Keywords:
nightmare, dreaming, emotional regulation, executive functions, Stroop task
1. Introduction
Idiopathic nightmare sufferers frequently – at least once a week – experience visually vivid, intense and disturbing dreams that involve fear, anxiety, anger, sadness, disgust or other unpleasant emotions (Nielsen & Zadra, 2010). According to the International Classification of Sleep Disorders, 2nd edition (ICSD-II, 2005) these dream disturbances end in abrupt awakenings. However, research on the nature of bad dreams (disturbing dreams that do not awaken the dreamer) suggests that the awakening criterion for nightmare disorder is unnecessarily narrow (Blagrove, Farmer, & Williams, 2004; Spoormaker, Schredl, & van den Bout, 2006; Zadra & Donderi, 2000). Others proposed that disturbed dreaming forms a continuum from normal dysphoric dreaming to post-traumatic nightmares where the pressure for awakening varies as a function of situational and dispositional factors as well (Levin, Fireman, Spendlove, & Pope, 2011; Levin & Nielsen, 2007).
Disturbed dreaming is associated with a variety of psychopathological conditions (Agargun et al., 2007; Besiroglu, Agargun, & Inci, 2005; Krakow et al., 2002; Roberts & Lennings, 2006; Semiz, Basoglu, Ebrinc, & Cetin, 2008). These findings provide valuable data on the comorbidity of mental disorders and dream disturbances, but cannot reveal the mechanisms and emergence of disturbed dreaming per se because of the confounding effects of waking pathology. While the psychiatric perspective assumes that nightmares and bad dreams are ‘‘mere’’ symptoms of an underlying mental disorder, recent findings suggest the nature of this relationship to be more complex (Lancee, Spoormaker, & van den Bout, 2010; Spoormaker & Montgomery, 2008). For instance, some studies have failed to detect a direct association between psychopathology and nightmare frequency (Levin & Fireman, 2002; Levin & Nielsen, 2007), especially when mental disorders were examined among a sample of frequent nightmare reporters (instead of the inverse, investigating nightmare frequency in psychiatric populations) (Lancee et al., 2010). The association between nightmares and mental complaints seems to be mediated by nightmare distress, the affective and cognitive impact of nightmares on daytime functioning (Belicki, 1992; Blagrove et al., 2004). Furthermore, nightmare frequency was shown to be a stable disposition with high genetic heritability, which was independent of the genetic influences of general waking anxiety (Coolidge, Segal, Coolidge, Spinath, & Gottschling, 2010).
These findings suggest that instead of the prevailing view of being a symptom of waking dysfunctions, frequent nightmares should be conceptualized as a specific sleep disorder (Spoormaker et al., 2006). Despite their relatively high prevalence (4–5%) in the general population (Nielsen & Zadra, 2000), the underlying mechanisms of nightmare disorder were only scarcely investigated (Germain & Nielsen, 2003; Nielsen, Paquette, Solomonova, Lara-Carrasco, Popova, et al., 2010; Nielsen, Paquette, Solomonova, Lara-Carrasco, Colombo, et al., 2010).
Recent findings indicate that sleep is intimately related to the processing and probably to the regulation of affect-laden memories (Walker, 2009). Studies examining sleep-dependent emotional memory consolidation indicate that emotional processing may especially benefit from REM sleep (Nishida, Pearsall, Buckner, & Walker, 2009; Wagner, Gais, & Born, 2001; Wagner, Hallschmid, Rasch, & Born, 2006). Moreover, REM sleep involves the intense activation of an emotional network comprising the amygdala, the anterior cingulate and the ventromedial prefrontal cortex (Desseilles et al., 2006; Maquet et al., 2005; Muzur, Pace-Schott, & Hobson, 2002). Nightmares generally – but not exclusively – occur during REM sleep (Spoormaker et al., 2006) suggesting that disturbed dreaming is an example of dysfunctional emotional processing during REM sleep. In their integrative model, Levin and Nielsen (2007) proposed that nightmares may be the consequence of the inefficient down-regulation of amygdalar over-activation, and a failure to provide new spat
iotemporal contexts for the fearful emotional memories processed during REM sleep. According to the model, the former dysfunction is related to impairments in the ventromedial prefrontal and the anterior cingulate cortex, while the latter is related to impaired hippocampal functioning. Furthermore, these impairments are related to the failure in creating adaptive fear-extinction memories leading to emotional dysregulation during sleep (Levin & Nielsen, 2007; Levin & Nielsen, 2009; Nielsen & Levin, 2007).
Recent findings on healthy subjects support the idea that sleep and especially REM sleep has an important role in the generalization and consolidation of fear-extinction memories, respectively (Pace-Schott et al., 2009; Spoormaker et al., 2010). Nevertheless, no prior studies have examined fronto-limbic abnormalities and/ or related executive functions in subjects with frequent nightmares. We hypothesized that if nightmare subjects were characterized by impaired prefrontal and fronto-limbic functions during REM sleep, alterations in these networks would be reflected during waking tasks as well. Therefore, the aim of our experiments was to test the neurocognitive model of Levin and Nielsen (2007) through different neuropsychological assessments of executive functions.
2. Study I
In order to examine the behavioral effects of impaired prefrontal and fronto-limbic functioning in subjects with frequent nightmares, we applied a series of neuropsychological tasks that were previously shown to rely on these brain areas. Executive functions or cognitive control processes involving the manipulation of items in working memory or the inhibition of prepotent but inappropriate response tendencies are considered to activate mainly prefrontal and related neural structures (Botvinick, Cohen, & Carter, 2004; Bush, Luu, & Posner, 2000; Dillon & Pizzagalli, 2007; Garavan, Ross, Murphy, Roche, & Stein, 2002; Rueda, Posner, & Rothbart, 2005). In case of emotional information, such functions seem to activate more specifically the ventrolateral and the ventromedial prefrontal, as well as the rostral anterior cingulate cortex (Bremner et al., 2004; Bush et al., 2000; Chiu, Holmes, & Pizzagalli, 2008; Lane et al., 1998; Wingenfeld et al., 2009).
In light of these findings and based on Levin and Nielsen’s (2007) neurocognitive model presuming fronto-limbic impairments as the neural background of disturbed dreaming, we anticipated that the nightmare (NM) group – in comparison with the controls (CTL) – would show worse performance in different executive tasks, and especially in those that require the processing of negative emotional information. In order to test this hypothesis we applied three well-characterized paradigms, the Emotional Go/NoGo task, the Emotional Stroop task and the Letter-and Category Fluency task.
The Go/NoGo task is a frequently used paradigm to assay motor response inhibition to perceptual stimuli (Aron et al., 2007). The task involves the presentation of a series of ‘‘Go’’ cues to which subjects have to press a button as quickly as possible, and ‘‘NoGo’’ cues that require the inhibition of this motor response. In the emotional version of this task (Reynolds & Jeeves, 1978), emotionally salient (e.g. happy and/or angry faces) perceptual stimuli are interspersed with emotionally neutral stimuli (neutral faces). The Emotional Go/NoGo task assesses response inhibition in the context of affective information processing, allowing the investigation of perturbations in emotional processing. Previous studies indicate that the Emotional Go/NoGo task activates the ventrolateral prefrontal cortex in relation to response inhibition, while the ventromedial prefrontal cortex and the rostral anterior cingulate cortex are activated in relation to the processing of negative emotional information (Chiu et al., 2008; Dolcos, Kragel, Wang, & McCarthy, 2006; Dolcos & McCarthy, 2006; Hare & Casey, 2005). Since the proper functioning of these networks are reflected by the behavioral measures of reaction time and accuracy (Hare & Casey, 2005; Waters & Valvoi, 2009), we hypothesized that NM subjects – in comparison with CTLs – would be characterized by longer reaction times and more false alarms, especially in the condition involving the inhibition of negative emotional information (Neutral Go/AngryNoGo).
The Emotional Stroop task is a widely used tool to investigate attentional bias and emotional interference caused by emotionally salient stimuli (MacLeod, Mathews, & Tata, 1986). The task involves the presentation of neutral and emotionally charged stimuli (e.g. neutral and emotionally negative words) with different colors, and participants are asked to press the button corresponding to the color of the word as quickly as possible. Since the semantic content of the words are irrelevant for the task, subjects may suppress distracting semantic representations, and focus only on the perceptual information (the color) of the presented words. Emotionally charged words may produce stronger interference, since they capture the attention more effectively than neutral words, and may also require additional cognitive processes in order to suppress the semantic content and also to regulate evoked emotional reactions. Consequently, reaction times are generally longer for affect-laden words, especially in subjects who are characterized by emotional dysregulation (Becker, Rinck, Margraf, & Roth, 2001; Bremner et al., 2004; Hope, Rapee, Heimberg, & Dombeck, 1990; Mattia, Heimberg, & Hope, 1993). Brain imaging studies indicate that the Emotional Stroop task is associated with enhanced activation in the amygdala, the anterior cingulate cortex and the middle frontal gyrus (Bremner et al., 2004; Bush et al., 2000; Whalen et al., 1998; Wingenfeld et al., 2009). In light of these findings, we expected that NM subjects – in contrast to CTLs – would exhibit worse performance in the Emotional Stroop task, reflected by longer reaction times and/or more errors in the trials involving negative emotional stimuli. In other words, we expected enhanced emotional interference in the nightmare in comparison with the control group.
The Letter-and Category Fluency task is a classical neuropsychological task generally used to measure executive functions in different clinical settings (Baldo & Shimamura, 1998; Baldo, Shimamura, Delis, Kramer, & Kaplan, 2001; Curtis et al., 1998; Lezak, 2004; Moritz et al., 2002). The task requires participants to generate as many different words as they can in 60 s, starting with a given letter (Letter Fluency) or belonging to a semantic category (Category Fluency). The task is considered to engage different cognitive processes such as working memory, cognitive flexibility and inhibitory control (Baldo, Schwartz, Wilkins, & Dronkers, 2006). Lower fluency and perseveration errors (repetitions) reflect executive dysfunctions. Performance in the Letter-and Category Fluency task was related to the frontal and the temporal cortex, respectively (Baldo et al., 2006), but other findings indicate that both areas are implicated in the letter-based and category-based word generation (Baldo & Shimamura, 1998; Schwartz, Baldo, Graves, & Brugger, 2003). Since the task is sensitive to a wide range of executive functions underlain by the prefrontal cortex, we anticipated worse performance in the NM group in contrast to CTLs.
Furthermore, since performance in these three tasks may be influenced by waking anxiety and sleep quality, we aimed to control the confounding effects of these factors as well.
2.1. Methods and materials
2.1. 1. Participants
Participants (all native Hungarians) were selected from a large pool of undergraduate students attending to different introductory psychology courses at the Budapest University of Technology and Economics. First they completed an on-line questionnaire assessing dream quality and a variety of personality factors. Findings on the relationship between dream quality and personality were reported (Simor, Köteles, & Bódizs, 2011) and will be reported elsewhere. Dreaming-related questionnaires included the Dream Quality Questionnaire (DQQ) (Bódizs, Simor, Csóka, Bérdi, & Kopp, 2008), the Hungarian version of the Van Dream Anxiety Scale (VDAS-H) (Agargun et al.,1999; Simor, Kovács, et al., 2009) and a 7-point Likert scale with two items; one assessing the frequency of nightmares with awakenings, and the other the frequency of bad dreams without awakenings (0 – Almost never; 1 – once or twice a year; 2 – every 2–3 month; 3 – once in a month; 4 – twice a month; 5 – once a week; 6 – more than once a week). Subjects of the nightmare group were selected on the basis of the International Classification of Sleep Disorders, 2nd edition criteria and Levin and Nielsen’s (2007) model of disturbed dreaming, including disturbed dreamers without abrupt awakenings. Moreover, after the first selection, participants kept a 2-week daily dream-log, and rated the emotional quality of their reported dreams based on the items of the DQQ (Bódizs et al., 2008). Subjects reporting one or more nightmares or bad dreams per week in the retrospective questionnaires and in the dream-logs were enrolled in the NM group, while individuals having less than two nightmares and bad dreams during the last year, and no nightmares and bad dreams at all in the 2 week dream-log period were enrolled in the CTL group. Subjects were thoroughly interviewed about their negative dream experiences and the content of their reported dreams. Those subjects who reported the onset of negative dream experiences in relation to a traumatic event or indicated that their reported dreams were related to a prior trauma (such as physical attack, accident, and sudden death of a close relative) were excluded from the study. Finally, 35 (21 female and 14 male) NM (Mage=20.5±1.8y) and 35 (21 female and 14 male) CTLs (Mage = 20.1 ± 1.34 y) were selected. (The difference in age was not significant between the two groups: F1,68 = 1.5 p = 0.23) None of the subjects reported prior neurological, psychiatric or sleep disorders or prior history of any chronic disease. NM subjects scored higher on the Nightmare Frequency Scale (NM: 5.52 ± 1.46 vs. CTL: 1.54 ± 0.66; F1,68=, p < 0.0001), on the Bad Dream Frequency Scale (NM: 5.61 ± 1.48 vs. CTL: 1.77 ± 0.8; F1,68=, p < 0.0001), on the Negative Dream Affect Scale of the DQQ (NM: 8.7 ± 0.75 vs. CTL: 4.4 ± 0.79; F1,68 = 557.03, p < 0.0001) and on the VDAS-H (NM: 19.5 ± 7.6 vs. CTL: 0.26 ± 0.78; F1,68 = 221.6, p < 0.0001), indicating at least moderately severe dream disturbances (Bódizs et al., 2008; Simor, Kovács, et al., 2009). The study protocol was approved by the local ethical review board of the Budapest University of Technology and Economics. Subjects were told that the aim of the study was the investigation of the relationship between dreaming and cognitive processes. The subjects received partial credit points in the introductory psychology course as compensation for their participation. Written informed consent was obtained.
2.1.2. Procedures
Subjects completed the psychometric measures and neuropsychological tasks in a noise-attenuated room. The order of the tasks was fixed for all subjects.
2.1.2.1. Psychometric measures. The Dream Quality Questionnaire (Bódizs et al., 2008) includes 11 items assessing the tendency of experiencing nightmares with recurrent or non-recurrent content; the vividness, bizarreness and emotional load of dreams; the effect of dreams on daytime mood and the frequency of having night-terror-like symptoms. The measure contains three main components: the negative, positive and the neutral emotional aspects of dreams. According to previous results the DQQ proved to be a valid instrument measuring the above qualities of dreaming (Bódizs et al., 2008). Since the present study focused on the aspects of disturbed dreaming, we only analyzed the data regarding the Negative Dream Affect Scale of the DQQ.
The Van Dream Anxiety Scale (VDAS) (Agargun et al., 1999) provides information about nightmare frequency and dream anxiety (nightmare distress) caused by frightening dreams. The items of the self-rating scale are concerned with nightmare frequency and the maleficent effects of nightmares on daytime functioning. Items are weighted on a 0–4 scale and summed to yield a global VDAS score of 0–52. The Hungarian version of the scale proved to be a reliable (a = 0.96) and valid instrument in order to measure dream anxiety (Simor, Kovács, et al., 2009). Internal consistency of the VDAS was excellent in previous studies (α = 0.91) (Simor, Köteles, Sándor, Petke, & Bódizs, 2011).
In order to control for the effects of sleep quality, the Hungarian version of the Groningen Sleep Quality Scale (GSQS) (Simor, Köteles, Bódizs, & Bárdos, 2009) was used. The 14-item questionnaire measures the extent of subjective sleep fragmentation by a binary scale. The internal reliability and validity measures of the scale indicated that the questionnaire was an adequate tool for assessing subjective sleep quality (Simor, Köteles, et al., 2009).
The STAI (Spielberger, Gorsuch, & Lusgene, 1970) is a widely used self-report instrument that differentiates between the temporary condition of state anxiety and the longstanding quality of trait anxiety. We used the 20-item Hungarian version of STAI trait anxiety questionnaire (STAI-T) in order to assess general levels of anxiety (Sipos, Sipos, & Spielberger, 1994). The questionnaire proved to be a valid tool for the measurement of trait anxiety, showing excellent internal consistency in different studies (Köteles et al., 2011).
2.1.2.2. Emotional Go/NoGo task. In a computerized version of the Emotional Go/NoGo task we used pictures of faces with neutral, angry and happy facial expressions. 56 faces were selected from multiple open-source face-databases and were rated previously by 30 subjects. (These subjects where different from our study participants.) They indicated which expressions they thought a particular face has and also estimated the typicality of that particular facial expression for the given emotion. We selected seven faces for every category with the best inter-rater agreement and highest typicality-ratings. These faces appeared on a screen against a black background during the task.
The task consisted of four blocks with 60 trials in each. In every trial, a randomly selected picture from one of the three facial-expression categories was presented. The four blocks differed in respect of which facial expression constituted a Go or a NoGo cue. In the first block during a Go trial a neutral face, whereas during a NoGo trial an angry face was presented. By contrast, in the second block neutral faces were designated as NoGo trials, and angry faces were set to Go trials. The design of the third and fourth block was analogous to the first two blocks except that here the angry faces were replaced by happy faces. Thus, in the four blocks, both angry and happy faces were associated with both Go and NoGo trials.
From the 60 trials in every block, 42 constituted a Go trial whereas 18 were NoGo trials. Every picture was presented for 1000 ms, and the pictures were separated by
a pause of 500 ms. Subjects were asked to give accurate responses as fast as possible. Subjects underwent a practice phase of 10 trials before the task. The order of the four conditions was randomized across the subjects.
2.1.2.3. Letter-and Category Fluency task. In the Letter Fluency task, participants had to generate as many words as they could in 60 s beginning with the letter F. They were asked to avoid repetitions of a previously mentioned word or word stem, and to avoid proper names. Subjects were subsequently asked to generate words beginning with the letters A and S. The words were written down by an assistant blind to the group membership of the subjects. In the Category Fluency task, subjects were given 60 s to generate words belonging to the semantic categories Hungarian male names, and then, Hungarian female names. Similarly, they were asked to avoid repetitions and nicknames of a previously mentioned name. Fluency was calculated by summing the number of the correct solutions. Perseveration was calculated by the number of repetitions/(number of correct + number of incorrect (repetitions, errors) responses).
2.1.2.4. Emotional Stroop task. During the Emotional Stroop task subjects were sitting in front of a computer screen, where different words were presented against a black background. The font color was randomly set to one of the following four colors: red, blue, green and yellow. Subjects had to indicate the color of the word – as fast as possible – by pressing a key on the keyboard assigned to the specific color. The task consisted of four blocks, with the same 30 words presented in each block following a fully randomized sequence, and the words were separated by a pause lasting randomly between 300 and 1200 ms.
Half of the words had intense negative valence, whereas the other half were neutral. Subjects practiced with 10 neutral words before performing the task. The words were selected from a 480 word pool, each rated by 54 subjects in a pilot study on the following dimensions: arousal, valence and dominance. (The subjects who rated the words were different from our study subjects). The selected 30 words were matched in respect to valence (neutral or negative) and word frequency.
2.1.2.5. Data analysis. Mean scores of the two groups (NM vs. CTL) in the GSQS and the STAI-T questionnaire were compared with the Welch test and with independent t-test, respectively. (We applied the Welch test if the criterion for the homogeneity of variance were violated.) Reaction times of the correct responses in the Emotional Go/NoGo task were compared using repeated measures ANOVAs with Group (NM, CTL) as a between-subject factor and Valence (angry vs. happy faces) as a within-subject factor. In order to measure the differences in accuracy, sensitivity scores (D-prime value: D’= Z(hit rate) – Z(false alarm rate) of the two groups in the Go/ NoGo task were compared with oneway ANOVA. To compare mean reaction times for correct responses in the Emotional Stroop task we used repeated measures ANOVA with Group as a between-subject factor and Valence (neutral vs. negative words) as a within-subject factor. The number of errors in the Emotional Stroop task between the two groups was compared with oneway ANOVA. To control the possible mediating effects of sleep quality and waking anxiety on the neuropsychological task performance, we also analyzed the same between-and within subject effects by using the GSQS and the STAI-T scores as covariates in the repeated measures ANCOVAs. In order to compare performance in the Letter-and Category Fluency task controlling for the effects of anxiety and sleep quality we applied a MANOVA analysis with Fluency and Perseveration scores as the dependent variables, Group as a between-subject factor, and subsequently a MANCOVA with the same variables but also with the STAI-T and GSQS score as covariates in the model.
2.2. Results
2.2.1. Psychometric tests
The NM group scored higher (M = 5.29 ± 3.2) than the CTL group (M = 2.06 ± 2.2) on the GSQS, showing worse subjective sleep quality (t60 = 24.37, p < 0.0001), and on the STAI-T, showing higher levels of dispositional anxiety (M = 49.2 ± 9.1 vs. M = 34.2 ± 8 t68 = 7.29, p < 0.0001).
2.2.2. Emotional Go/NoGo task
In the condition where emotional stimuli were the targets and neutral stimuli the distractors (Angry Go/Neutral NoGo vs. Happy Go/Neutral NoGo) a significant main effect of Valence emerged (F1,68 = 47.67, p < 0.0001) due to slower reaction times (ms) for Angry targets vs. Happy targets (Mangry targets = 472.2 ± 71.36 vs. Mhappy targets = 425 ± 55.73). Neither the effect of Group (NM vs. CTL) nor the interaction of Group x Valence reached significance (F1,68 = 0.96, p = 0.34; F1,68 = 0.01, p = 0.92; respectively). After controlling for the effects of sleep quality (using the GSQS as covariate), the effect of Valence (F1,67 = 15.7, p < 0.0001) remained significant; but after controlling for trait anxiety (using the STAIT as the only covariate), Valence showed only a trend (F1,67 = 3.66, p = 0.06). The main effect of Group and the interaction between Group and Valence remained non-significant after controlling for sleep quality (F1,67 = 1.43, p = 0.31; F1,67 = 0.19, p = 0.89; respectively) or trait anxiety (F1,67 = 0.7, p = 0.79; F1,67 = 0.09, p = 0.77; respectively).
In the condition involving inhibition in response to emotional stimuli (Neutral Go/Angry NoGo vs. Neutral Go/Happy NoGo) a significant effect of Valence emerged (F1,68 = 11.53, p = 0.001) due to longer reaction times for Neutral targets with Angry distractors vs. Neutral targets with Happy distractors (Mangry distractors = 485.24 ± 62.02 vs. Mhappy distractors = 467.08 ± 55.35).
The main effect of Group yielded a trend difference (F1,68 = 3.78, p = 0.056) due to longer reaction times in the NM (Mangry distractors = 497.32 ± 64.19; Mhappy distractors = 479.64 ± 52.69) compared to the CTL group (Mangry distractors = 472.59 ± 58.03; Mhappy distractors = 454.13 ± 55.79). Contrary to our hypothesis, the interaction between Group and Valence was not significant (F1,68 = 0.01, p = 0.93). After controlling for sleep quality, the main effect of Valence (F1,67 = 3.89, p = 0.053) and Group (F1,67 = 3. 52, p = 0.064) showed a trend, but after controlling for anxiety, both effects became nonsignificant (F1,67 = 0.07, p = 0.74; F1,67 = 1.04, p = 0.31; respectively).
In order to compare the sensitivity index between NM and CTL group, we compared the mean D-prime (D’) values between the two groups. Mean scores of D’ values (that were very high, ranging from 3.5 to 3.9 in the different conditions) for each condition were not significantly different between the NM and the CTL group (F1,68 = 0.24, p = 0.62; F1,68 = 1.11, p = 0.29; F1,68 = 0.01, p = 0.94; F1,68 = 0.1, p = 0.92).
2.2.3. Emotional Stroop task
In the Emotional Stroop task there was a significant main effect of Valence (F1,68 = 7.14, p < 0.01) due to longer mean reaction times (in ms) in response to negative (M = 688.3 ± 59. 06) in contrast to neutral words (M = 678.76 ± 77. 53). NM group showed significantly (F1,68 = 10.39, p = 0.002) longer reaction times (Mneutral = 707.14 ± 86.44; Mnegative = 709.58 ± 60.67) than the CTL group for both types of stimuli (Mneutral = 651.19 ± 56.41; Mnegative = 667.63 ± 50.1) (see Fig. 1). The interaction of Group x Valence showed a marginal effect (F1,66 = 3.92, p = 0.052). Parsing of this interaction (by ex
amining the performance of the two groups separately) revealed a significant within-subject effect of Valence in the CTL group (F1,34 = 31.96, p < 0.0001), but not in the NM group (F1,34 = 0.14, p = 0.71). Contrary to our expectations, mean reaction times in the NM group were not different for neutral and negative words. In contrast, the CTLs were faster in response to neutral words than to negative words. After controlling for sleep quality and/or anxiety (by using GSQS and subsequently STAI-T as covariates), the main effect of Group remained signi.cant (F1,66 = 6.11, p = 0.016) and the interaction of Group x Valence showed a significant effect (F1,66 = 5.2, p = 0.026). In contrast, the effect of Valence was no longer significant (F1,66 = 0.19, p = 0.89). The effect of Valence was not significant even after controlling for sleep quality and anxiety independently. Nevertheless, the interaction of Valence x sleep quality was significant (F1,66 = 4.15, p = 0.04), indicating longer reaction times for negative words in case of more fragmented sleep. The mean number of errors (ranging between 0.9 and 1.2) of the neutral and the negative trials in the Emotional Stroop task did not differ significantly between the NM and CTL group (F1,68 = 0.18, p =0.67; F1,68 =0.49 p = 0.49, respectively).
2.2.4. Letter-and Category Fluency task
The between subject Group had a significant main effect on Perseveration scores (F1,68 = 11.47, p < 0.001) because of the significantly higher scores of perseveration errors in the NM (M = 0.4 ± 0.32) compared with the CTL group (M = 0.18 ± 0.23). Regarding Fluency the effect of Group was not significant (F1,68 = 0.04, p = 0.85). After controlling for trait anxiety and sleep quality, the effect of Group on Perseveration remained significant (F1,66 = 7.3, p = 0.009) and Fluency showed a trend difference between the groups (see Table 1). While sleep quality was not related to the performance in the Fluency task, higher levels of trait anxiety were associated with lower fluency scores. (Subsequent analysis indicated that the STAI-T scores correlated negatively with the Fluency scores: Pearson r = -0.26, p = 0.03).

Fig. 1. Reaction times in the mixed design Emotional Stroop task by Group and Valence (neutral vs. negative stimuli). The NM group was significantly slower in both conditions (p = 0.002). While in the CTL group a significant difference occurred in response to neutral and negative words (p < 0.0001), the NM group did not differ in those (p = 0.71).
Table 1
Results of the multivariate analysis of variance of the letter-and category fluency task. Tests of between-subjects effects. Independent variables: Group (NM = 1, CTL = 2), STAI-T (Spielberger Trait Anxiety Inventory), GSQS (Groningen Sleep Quality Scale).
| Independent variables |
Dependent variables |
F-value |
Significance |
| Group |
Fluency |
3.39 |
p = 0.07 |
| |
Perseveration |
7.3 |
p = 0.009 |
| STAI-T |
Fluency |
6.04 |
p = 0.017 |
| |
Perseveration |
0.56 |
p = 0.46 |
| GSQS |
Fluency |
0.57 |
p = 0.46 |
| |
Perseveration |
0.26 |
p = 0.61 |
2.3. Discussion of Study I
In our first study we aimed to characterize executive control processes in a group of young subjects with frequent nightmares and healthy controls by two tasks involving affect-laden stimuli (Go/NoGo and Stroop) and one without emotional information. We showed that NM subjects by comparison with the CTL group exhibited worse performance in these executive tasks. Moreover, lower performance in these tasks cannot be attributed exclusively to the effects of waking anxiety or disturbed sleep.
In the Emotional Go/NoGo task, subjects of both groups showed increased response time when the stimuli included negative emotional information (angry faces) as targets, as well as distractors; suggesting that identifying or suppressing threatening stimuli requires additional attentional resources in comparison with neutral or positive emotional stimuli.
The performance of the NM and CTL group did not differ regarding identifying emotional faces among neutral distractors, but NM subjects exhibited slightly increased response time to neutral target faces embedded among emotional distractors. This finding dovetails with previous studies indicating that this identifying process requires enhanced attentional resources (Chiu et al., 2008), and resembles the findings of a similar study examining children with anxiety disorders (Waters & Valvoi, 2009). Interestingly, this relative slowing in the NM group was also present in case of neutral targets embedded among positive (happy faces) distractors. This indicates that the lower performance of disturbed dreamers was independent of the valence of the distractors. Nevertheless, the general slowing pattern in NM subjects was influenced by trait anxiety but not by poor sleep quality.
Accuracy did not differ between NM and CTL subjects, probably because the task was relatively easy for the subjects as evidenced by the high D-prime values.
In sum, the results of the Emotional Go/NoGo task partially supported our first hypothesis anticipating worse performance in NM in comparison with CTL subjects. While accuracy was not different in any condition between the two groups, NM subjects were slightly slower in responding to neutral stimuli among emotional distractors, suggesting that individuals with frequent nightmares require additional cognitive efforts for emotion-modulated response inhibition. Nevertheless, the disadvantage for NM subjects disappeared if we controlled the effects of trait anxiety, suggesting that not nightmare disorder per se, but the higher levels of waking anxiety are associated to weaker performance in the Emotional Go/ NoGo task.
In accordance with previous studies (Becker et al., 2001; Hope et al., 1990; Mattia et al., 1993), we found significantly longer reaction times for negative words in contrast to neutral words in the Emotional Stroop task. However, only CTL subjects showed this relative slowing in response to negative words, whereas NM subjects were characterized by similar reaction times for negative and neutral words contrary to our expectations. The NM in comparison with the CTL group showed significantly longer reaction times for negative and neutral words as well. This finding partially supports our second hypothesis claiming longer reaction times in NM subjects, but the specific sensitivity of NM subjects in response to negative words was not supported. On the contrary, NM subjects exhibited an overall slowing pattern in the Emotional Stroop task, irrespective of the valence of the stimuli. Furthermore, this slowing was not a function of trait anxiety or disturbed sleep, but an independent characteristic of the NM group. This finding is in accordance with previous studies examining individuals with Borderline Personality Disorder (Wingenfeld et al., 2009) and Post-traumatic Stress Disorder (Bremner et al., 2004) – two conditions associated with disturbed dreaming (Mellman, David, Bustamante, Torres, & Fins, 2001; Simor, Csóka, & Bódizs, 2010) – showing similar patterns of overall slowing in Emotional Stroop paradigms. Nevertheless, our findings indicating emotional interference in CTL but not in NM subjects still require further considerations. We assumed tha
t the lack of emotional interference in the NM group was due to the design of our Emotional Stroop task. Since we applied a mixed set of neutral and negative words that were presented randomly in the same block, a slow effect of emotional interference might have confounded our results. Emotionally charged, negative words may exert a slow effect, and thus, interfere with the processing of subsequent neutral trials as well (McKenna & Sharma, 2004; Phaf & Kan, 2007). Therefore, the emotional interference caused by the negative words might have increased the response time not only for the negative but for some neutral words as well. If NM subjects were specifically sensitive to negative emotional information, in this group, a slow effect of emotional interference might have influenced response time for the neutral trials as well. Given these considerations, in our second study (see Section 3) we applied an Emotional Stroop task with block design.
In contrast to reaction times, accuracy did not differ between the two groups, both committing a low number of errors.
Finally, we found that poor sleep quality was related to less efficient processing of negative words. This finding is in accordance with previous studies showing increased sensitivity for emotional information in sleep deprived subjects (Franzen, Buysse, Dahl, Thompson, & Siegle, 2009; Tempesta et al., 2010). Increased sensitivity and attentional bias for negative words might require additional cognitive resources in order to suppress the semantic content of the stimuli.
In sum, NM subjects showed impaired performance in the Emotional Stroop task, reflected by an overall increase in response time. This general slowing was not explained by trait anxiety or by disturbed sleep. Nevertheless, the lack of emotional interference in the NM group called for further investigations.
Results of the Letter-and Category Fluency task supported our third hypothesis expecting worse performance in NM compared to CTL subjects. NM subjects, compared to CTLs were characterized by slightly less fluent word generation and a notably higher number of perseverations, even after controlling for the effects of sleep quality and trait anxiety. While trait anxiety was associated to lower performance in word generation, the higher number of perseverations in the NM group was independent of the effects of anxiety.
3. Study II
In our first study NM subjects in comparison with CTLs exhibited longer reaction times in the Emotional Stroop task irrespective of the emotional nature of the stimuli. Nevertheless, the presentation of neutral and negative words in mixed design might have confounded our results due to the slow effect of negative emotional stimuli (McKenna & Sharma, 2004). In order to test this assumption we examined a new nightmare group and healthy controls by using an Emotional block design Stroop task. We hypothesized that both groups would be slower in response to negative words than to neutral ones, but NM subjects would exhibit increased emotional interference (increased response time for negative vs. neutral words) in comparison with CTLs.
Moreover, in order to test if NM subjects show lower performance in the Stroop task regardless of the emotional nature of the stimuli, we applied the Color-word Stroop task (Stroop, 1992). This classic Stroop task has been widely used for evaluating executive functions (Leung, Skudlarski, Gatenby, Peterson, & Gore, 2000; Zysset, Müller, Lohmann, & Von Cramon, 2001). In the Color-word Stroop task subjects are required to press a button corresponding to the color of the stimuli. In the incongruent condition, subjects are asked to press the button corresponding to the color of the stimulus which is a color word different from the stimulus (e.g. the word ‘‘blue’’ in yellow font); in the congruent condition the color word is presented with a matching color; and in the neutral condition subjects are asked to respond to a control (e.g. XXXXX) stimulus. In the incongruent condition the semantic representation of the word must be inhibited in favor of the perceptual characteristic of the stimulus. Brain imaging studies of the Color-word Stroop task indicate that the conflict resolution in this condition is associated with enhanced activation in the dorsolateral prefrontal cortex and in the dorsal anterior cingulate cortex (Banich et al., 2000; Bush et al., 2000; Milham & Banich, 2005; Milham et al., 2002). Since the task requires executive functions relying on pre-frontal structures, we anticipated that NM subjects – compared to CTLs – would exhibit worse performance in the incongruent condition of the Color-word Stroop task. Similarly to Study 1, we controlled the effects of trait anxiety and sleep quality.
3.2. Methods
3.1.1. Participants
19 (7 female and 12 male) NM (Mage = 19.9 ± 1.3 y) and 17 (8 female 9 male) CTL subjects (Mage = 19.8 ± 1.3 y) were selected by the procedure described in study 1 (see Section 2.1.1). (Both groups comprised a new sample as none of these subjects participated in Study 1.) The difference in male–female ratio was not significant between the groups (Χ2 = 0.39, p = 0.53). None of the subjects reported having prior neurological, psychiatric or sleep disorders or prior history of any chronic disease. None of them reported traumatic experiences such as physical attack, accident, and the sudden death of a close relative. NM subjects scored higher on the Nightmare Frequency Scale (NM: 4.21 ± 1.84 vs. CTL: 1.59 ± 0.71; F1,34 = 30.28, p < 0.0001), on the Bad Dream Frequency Scale (NM: 5.74 ± 1.24 vs. CTL: 1.65 ± 0.86; F1,34 = 128.96, p < 0.0001), on the Negative Dream Affect Scale of the DQQ (NM: 9.02 ± 1.47 vs. CTL: 5.5 ± 0.94; F1,34 = 71.63, p < 0.0001), and on the VDAS-H (NM: 15.1 ± 9.34 vs. CTL: 0.29 ± 0.85; F1,34 = 42, p < 0.0001), indicating at least moderately severe dream disturbances (Bódizs et al., 2008; Simor, Kovács, et al., 2009).
The study protocol was approved by the local ethical review board of the Budapest University of Technology and Economics. Subjects were told that the aim of the study was the investigation of the relationship between dreaming and cognitive processes. The subjects received partial credit points in the introductory psychology course as compensation for their participation. Written informed consent was obtained.
3.1.2. Procedure
Subjects completed the Groningen Sleep Quality Scale (GSQS), the STAI-T questionnaire (see Section 2.1.2.1) and the Stroop tasks in a noise-attenuated room. The order of the tasks (first the Color-word and then the Emotional Stroop task) was fixed for all subjects.
3.1.2.1. Color-word Stroop task. We used a computerized version of the Color-word Stroop task. There were three conditions: neutral, congruent and incongruent. In all conditions words or letters were presented with various font colors, and subjects had to indicate the color of the stimuli by pressing – as fast as possible – one of the four predefined keys.
The task consisted of three blocks after a short practice phase. In each block, there were 32 trials of the control condition (a row of five ‘‘Xs’’), followed by 32 trials of the congruent (e.g. ‘‘RED’’ with red font color), and 32 trials of the incongruent condition (e.g. ‘‘RED’’ with blue font color). This yielded a total of 96 trials for all three conditions. The trials were separated by a random pause that lasted between 300 and 1200 ms.
3.1.2.2. Emotional Stroop task (Block design). The Emotional Stroop task was similar to the one used in Study 1. We presented subjects with neutral and neg
ative words with different font colors, and they had to indicate the color of the words by pressing a predefined key on the keyboard. However, in contrast to Study 1, we increased the number of the presented words to 32 neutral and 32 negative words. Moreover, we used a block design: first, 32 neutral words were presented which were followed by 32 negative words. This sequence was repeated three times. The order of the 32–32 neutral and negative words was randomized in every block, but the 32 neutral words came always before the 32 negative words. The duration of the inter-trial pause was set to a random value between 300 and 1200 ms.
3.1.2.3. Data Analysis. Mean reaction times for correct responses in the Emotional Stroop task were compared with repeated measures ANOVA with Group as a between-subject factor and Valence (Neutral vs. Negative) as a within subject factor. Similarly, in the Color-word Stroop task Group served as a between-subject factor and Condition (Control vs. Congruent vs. Incongruent) as a within-subject factor. The number of errors between the two groups was compared by oneway ANOVA. To control the possible mediating effects of sleep quality and waking anxiety on the neuropsychological task performance, we also analyzed the same between-and within subject effects by using the GSQS and the STAI-T scores as covariates in the repeated measures ANCOVAs. Since the set of stimuli in the Emotional Stroop task in contrast to the Color-word Stroop task includes longer words (e.g. bicycle vs. red), differences in word reading abilities may influence reaction times. Therefore, in order to control the confounding effects of word reading speed, a repeated measure ANOVA was used with Group as a between-subject factor, and Word length (words with two vs. three syllables) as a within-subject factor.
3.3. Results
3.2.1. Psychometric tests
The NM group scored higher (M = 4.32 ± 3.25) than the CTL group (M = 2.65 ± 2.37) on the GSQS, but the difference was not significant (t34 = 1.74, p = 0.086). STAI-T scores were significantly higher in the NM group (M = 45.63 ± 8.97 vs. M = 38 ± 11.8; t34 = 2.19, p = 0.036).
3.2.2. Color-word Stroop task
In the Color-word Stroop task a significant effect of Condition (F2,33 = 56.07, p < 0.0001) emerged due to longer reaction times (ms) for Incongruent (M = 797.25 ± 105.03) vs. Congruent (M = 717.07 ± 103.44; F1,34 = 61.96, p < 0.0001) and Incongruent vs. Control (M = 701.7 ± 109.57; F1,34 = 79.85, p < 0.0001) trials. Although NM subjects were slightly slower in each condition, the effect of Group was not significant (F2,32 = 2.04, p = 0.16; F1,32 = 2.18, p = 0.15, respectively). Similarly, the interaction between Group and Condition did not reach significance. The main effect of Condition remained significant after controlling for sleep quality (F2,32 = 18.14, p < 0.0001) or trait anxiety (F2,32 = 4.06, p < 0.027). CTL subjects committed more errors in the Incongruent condition compared to NM subjects (M = 2.12 ± 2.5 vs. M = 0.58 ± 0.96; F1,34 = 6.2, p = 0.018), while there were no signi.cant differences in the other two conditions regarding the number of errors.
3.2.3. Emotional Stroop task
In the Emotional Stroop task a significant effect of Valence emerged due to longer reaction times (ms) in response to negative words than to neutral words (M = 731.93 ± 110.82 vs. M = 709.52 ± 110.52; F1,32 = 25.48, p < 0.001). The effect of Group was also significant (F1,34 = 4.18, p = 0.049) because of longer reaction times in the NM group for both neutral and negative words (MNeutral = 743.56 ± 113.36; MNegative = 768.09 ± 109.43) compared with the CTL group (MNeutral = 665.12 ± 93.87; MNegative = 691.53 ± 100.53) (see Fig. 2). Contrary to our expectation, the interaction between Group and Valence was not significant, since both groups were significantly slower in response to negative than to neutral words (NM: F1,18 = 14.29, p = 0.001; CTL: F1,16 = 11.39, p = 0.004). After controlling for the effects of sleep quality, the effects of Valence (F1,33 = 8.43, p = 0.007) and Group (F1,33 = 4.96, p = 0.033) remained significant; but after controlling for the effects of trait anxiety the effect of Valence lost its significance (F1,33 = 0.09, p = 0.768), while the main effect of Group remained significant (F1,33 = 4.18, p = 0.049). There were no significant differences regarding the number of errors between NM and CTL subjects.
3.2.4. Word reading
Neither the main effect of Word length (F1,34 = 0.001, p = 0.974), nor the interaction between Group and Word length (F1,34 = 1.05, p = 0.312) had a significant effect on response time.
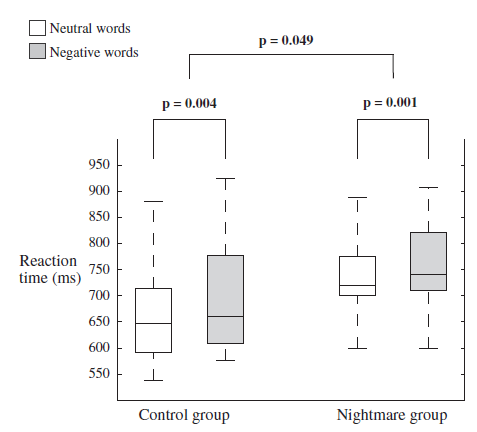
Fig. 2. Reaction times in the block design Emotional Stroop task by Group and Valence (neutral vs. negative stimuli). The NM group was significantly slower in both conditions (p = 0.049). In both groups subjects responded significantly slower to negative words (CTL: p = 0.004; NM: p = 0.001).
3.3. Discussion of Study 2
In coherence with our previous findings ( Study 1), results of Study 2 revealed increased reaction times in the NM as compared to the CTL subjects in the Emotional Stroop task. While in the Emotional Stroop task with mixed design (Study 1) NM subjects did not show emotional interference, in the blocked version (Study 2) both groups were characterized by longer reaction times in response to negative stimuli. This discrepancy suggests that the lack of emotional interference in the NM group of Study 1 might have been masked by the influence of negative stimuli on subsequent responses to neutral trials. Nevertheless, in the blocked version, the NM group exhibited longer reaction times in response to neutral words as well, showing an overall slowing pattern in the Emotional Stroop task, irrespective of the emotional nature of the stimuli. Therefore, contrary to our expectation, NM subjects were not characterized by increased emotional interference. Moreover, slower reactions for negative vs. neutral words (emotional interference) in both groups disappeared after the statistical control of trait anxiety, whereas the overall slowing in NM subjects was independent of poor sleep quality or trait anxiety.
In contrast to the findings in the Emotional Stroop task, NM subjects were not characterized by worse performance in the Color-word Stroop task, since mean reaction times did not significantly differ between the two groups in either condition. Indeed, CTLs in contrast to NM subjects exhibited more errors in the incongruent condition, the task requiring enhanced cognitive control in order to suppress the interfering semantic representation of the color word.
4. Discussion and conclusions
While in the last decades nightmare disorder was mainly investigated and substantially characterized from a clinical point of view, the mechanisms and the neurocognitive aspects of this disorder were only scarcely investigated (Germain & Nielsen, 2003; Levin & Nielsen, 2007; Nielsen, Paquette, Solomonova, Lara-Carrasco, Popova, et al., 2010; Nielsen, Paquette, Solomonova, Lara-Carrasco, Colombo, et al., 2010; Spoormaker, 2008). Levin and Nielsen (2009) provided a neurocognitive framework with testable hypotheses in order to model the mechanism of disturbed dreaming. They proposed that impaired prefrontal and fronto-limbic functions unable to regulate emotional activation during REM sleep are one o
f the crucial aspects of disturbed dreaming. The assumption of the impaired neural network in NM subjects was derived from the integration of previous research on emotional information processing in waking and brain imaging findings on REM sleep (Levin & Nielsen, 2007; Levin & Nielsen, 2009; Nielsen & Levin, 2007).
In the present studies we applied four different, well-characterized neuropsychological tasks that were shown to be associated with prefrontal and fronto-limbic functioning. We hypothesized that impaired prefrontal and fronto-limbic functions in relation to emotional dysregulation would be reflected in waking tasks as well, especially in those that require the processing of emotional information.
NM subjects exhibited lower performance in the emotional executive tasks, reflected by slightly increased response time in the Emotional Go/NoGo task involving emotional stimuli as dis-tractors, and by increased response time in the Emotional Stroop task. While the slowing pattern in the Emotional Go/NoGo seemed to be the result of higher levels of trait anxiety in the NM group, the slowing in the Emotional Stroop task was not related to anxiety. This latter finding is in coherence with recent findings indicating that disturbed dreaming is not a ‘‘mere symptom’’ of waking psychopathology (Coolidge et al., 2010; Lancee et al., 2010; Spoor-maker & Montgomery, 2008). Interestingly, lower performance in NM subjects in comparison with CTLs was independent of the emotional valence of the stimuli. Nightmare subjects exhibited longer response times in the Emotional Go/NoGo in case of positive emotional distractors and also in case of neutral words in the Emotional Stroop task, suggesting that impaired executive functions were not restricted to the processing of negative emotional information. These findings resemble earlier results examining executive functions in the context of affective stimuli in other populations characterized by emotional dysregulation (Bremner et al., 2004; Waters & Valvoi, 2009; Wingenfeld et al., 2009).
NM subjects showed impaired performance in the Letter-and Category Fluency task reflected by slightly lower fluency and a considerably higher number of perseverations. While the task aims to measure executive functions without involving emotional components, we suppose that even so, the task may evoke emotional reactions that might interfere with optimal performance. Although the task is apparently easy, according to our results it is rather difficult; moreover, subjects may get puzzled by their unexpectedly low performance. Even though earlier reports suggest that performance in Verbal Fluency tasks is not related to conditions of emotional dysregulation (Airaksinen, Larsson, & Forsell, 2005; Airaksinen, Larsson, Lundberg, & Forsell, 2004; Gruzelier, Seymour, Wilson, Jolley, & Hirsch, 1988), we found that fluency was related to higher levels of anxiety. Nevertheless, earlier investigations reporting the lack of associations between affective processes and fluency examined populations with severe psychopathological conditions, while our subjects comprised healthy university students and NM subjects without a prior history of mental disorders. We speculate that subclinical levels of trait anxiety influenced the rate of word generation due to performance anxiety and the unexpected difficulties of the task. However, it is also possible that other uncontrolled factors related to anxiety influenced task performance. While fluency was associated to trait anxiety, NM subjects exhibited a higher number of perseveration errors independently of the effects of anxiety, demonstrating that disturbed dreamers are characterized by prefrontal, executive deficits (especially dysfunctional inhibitory control).
NM subjects did not exhibit worse performance in the Color-word Stroop task. This executive task is associated with enhanced activation of the dorsolateral prefrontal and of the dorsal anterior cingulate cortex (Banich et al., 2000; Bush et al., 2000; Milham & Banich, 2005; Milham et al., 2002), structures that were not implicated in emotional regulation and in the mechanism of disturbed dreaming. Moreover, we speculate that in contrast to the Letter-and Category Fluency task – where subjects have to generate words aloud, in front of an assistant – the computerized Color-word Stroop task does not trigger negative emotional or cognitive reactions due to test or social anxiety. In line with this assumption, performance in the Color-word Stroop task was not influenced by trait anxiety scores.
In sum, our results support the assumption of executive dysfunctions in subjects with frequent nightmares, as evidenced by impaired performance in several neuropsychological tasks that involve the processing of emotional information, as well as the regulation of emotional reactions. We should note, however, that the overall slowing pattern of NM subjects in the Emotional Stroop task calls for further investigation, since this group was slower in response to neutral stimuli (neutral words) as well, that apparently do not involve emotional components. In contrast, they were not impaired in either condition of the Color-word Stroop task. NM subjects might have been slower in response to the neutral words because of slower word-reading, since these stimuli were generally longer than the color words (e.g. bicycle vs. red). However, this explanation seems unlikely since response time was not related to word length.
Another explanation is that NM subjects in comparison with controls are more sensitive to distracting neutral stimuli that elicit a broader network of semantic associations. Different, emotionally neutral words, such as trumpet, river, novel, and mirror might elicit a broad range of semantically related representations compared to the repeating presentation of the words: red, green, blue and yellow. These, ‘‘internal associations’’ may involve personally relevant semantic representations that interfere with the representation of the color of the stimuli. We speculate that subjects with frequent nightmares were more prone to be distracted by the spontaneous generation of semantic representations elicited by the words presented on the computer screen. This would partly explain lower performance in the Fluency task as well, since strategic (letter or category based) word generation relies on the suppression of semantically related spontaneous representations interfering with the task (Perret, 1974). This is also consistent with Hartmann and colleagues’ (1991) findings that individuals with thin boundaries – a psychological construct related to nightmare frequency and to more bizarre dream images – are characterized by a tendency toward immersion in internally generated associations and imaginative processes (Hartmann, 1989; Hartmann et al., 1991; Kunzendorf, Hartmann, Cohen, & Cutler, 1997). While this explanation seems plausible, it is not clear whether the general slowing may be the result of the above described hyperassociative process or the emotional context elicited by the self-relevant associations. Therefore, these considerations require further investigations.
Regarding the limitations of our study we should note that while we applied well characterized neuropsychological paradigms in order to examine prefrontal and fronto-limbic functioning in subjects with frequent nightmares, our results are only based on behavioral data that do not provide a precise picture about the underlying neural networks that are supposed to be dysfunctional in disturbed dreamers. Brain imaging or sophisticated electrophysiological methods would facilitate our understanding about altered fronto-limbic networks in nightmare reporters. Furthermore, examining a student sample is advantageous because of the relative homogeneity of our subjects, but care should be taken in generalizing our results to other populations. We should also note that other uncontrolled psychological variables such as d
epression might underlie the general slowing in the NM group. And finally, while we controlled for subjective sleep quality; more objective measures (e.g. polysomnography) of sleep architecture would be necessary in order to rule out the possible confounding effects of disrupted sleep on information processing.
In spite of these limitations, to the best of our knowledge this is the first investigation examining neuropsychological functions in subjects with frequent nightmares, and providing empirical data regarding the neurocognitive aspects of disturbed dreaming.
Acknowledgements
The research was supported by the 2010 Research Grant of the BIAL Foundation (55/10) and the 2009 Research Grant Award of the Joint IASD/DreamScience Foundation. The authors acknowledge Dr. Ferenc Köteles for his valuable comments on the manuscript of this article.
References
Agargun, M. Y., Besiroglu, L., Cilli, A. S., Gulec, M., Aydin, A., Inci, R., et al. (2007). Nightmares, suicide attempts, and melancholic features in patients with unipolar major depression. Journal of Affective Disorders, 98(3), 267–270.
Agargun, M. Y., Kara, H., Bilici, M., Sava, A., Telci, M., Semiz, B., et al. (1999). The van dream anxiety scale: A subjective measure of dream anxiety in nightmare sufferers. Sleep Hypnosis, 4, 204–211.
Airaksinen, E., Larsson, M., & Forsell, Y. (2005). Neuropsychological functions in anxiety disorders in population-based samples: Evidence of episodic memory dysfunction. Journal of Psychiatric Research, 39(2), 207–214.
Airaksinen, E., Larsson, M., Lundberg, I., & Forsell, Y. (2004). Cognitive functions in depressive disorders: Evidence from a population-based study. Psychological Medicine, 34(1), 83–91.
American Academy of Sleep Medicine, Task Force Chair HP. ICSD-II. (2005). International classification of sleep disorders: Diagnostic and coding manual (2nd ed.). Chicago: American Academy of, Sleep Medicine.
Aron, A. R., Durston, S., Eagle, D. M., Logan, G. D., Stinear, C. M., & Stuphorn, V. (2007). Converging evidence for a fronto-basal-ganglia network for inhibitory control of action and cognition. Journal of Neuroscience, 27(44), 11860–11864.
Baldo, J. V., Schwartz, S., Wilkins, D., & Dronkers, N. F. (2006). Role of frontal versus temporal cortex in verbal fluency as revealed by voxel-based lesion symptom mapping. Journal of the International Neuropsychological Society, 12(06), 896–900.
Baldo, J. V., & Shimamura, A. P. (1998). Letter and category fluency in patients with frontal lobe lesions. Neuropsychology, 12(2), 259–267.
Baldo, J. V., Shimamura, A. P., Delis, D. C., Kramer, J., & Kaplan, E. (2001). Verbal and design fluency in patients with frontal lobe lesions. Journal of the International Neuropsychological Society, 7(5), 586–596.
Banich, M. T., Milham, M. P., Atchley, R., Cohen, N. J., Webb, A., Wszalek, T., et al. (2000). FMRI studies of Stroop tasks reveal unique roles of anterior and posterior brain systems in attentional selection. Journal of Cognitive Neuroscience, 12(6), 988–1000.
Becker, E. S., Rinck, M., Margraf, J., & Roth, W. T. (2001). The Emotional Stroop effect in anxiety disorders: General emotionality or disorder specificity? Journal of Anxiety Disorders, 15(3), 147–159.
Belicki, K. (1992). Nightmare frequency versus nightmare distress: Relations to psychopathology and cognitive style. Journal of Abnormal Psychology, 101(3), 592–597.
Besiroglu, L., Agargun, M. Y., & Inci, R. (2005). Nightmares and terminal insomnia in depressed patients with and without melancholic features. Psychiatry Research, 133(2–3), 285–287.
Blagrove, M., Farmer, L., & Williams, E. (2004). The relationship of nightmare frequency and nightmare distress to well-being. Journal of Sleep Research, 13(2), 129–136.
Bódizs, R., Simor, P., Csóka, S., Bérdi, M., & Kopp, M. S. (2008). Dreaming and health promotion: A theoretical proposal and some epidemiological establishments. European Journal of Mental Health, 3(1), 35–62.
Botvinick, M. M., Cohen, J. D., & Carter, C. S. (2004). Conflict monitoring and anterior cingulate cortex: An update. Trends in Cognitive Sciences, 8(12), 539–546.
Bremner, J. D., Vermetten, E., Vythilingam, M., Afzal, N., Schmahl, C., Elzinga, B., et al. (2004). Neural correlates of the classic color and Emotional Stroop in women with abuse-related posttraumatic stress disorder. Biological Psychiatry, 55(6), 612–620.
Bush, G., Luu, P., & Posner, M. I. (2000). Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences, 4(6), 215–222.
Chiu, P. H., Holmes, A. J., & Pizzagalli, D. A. (2008). Dissociable recruitment of rostral anterior cingulate and inferior frontal cortex in emotional response inhibition. Neuroimage, 42(2), 988–997.
Coolidge, F. L., Segal, D. L., Coolidge, C. M., Spinath, F. M., & Gottschling, J. (2010). Do nightmares and generalized anxiety disorder in childhood and adolescence have a common genetic origin? Behavior Genetics, 40(3), 349–356.
Curtis, V. A., Bullmore, E. T., Brammer, M. J., Wright, I. C., Williams, S. C. R., Morris, R. G., et al. (1998). Attenuated frontal activation during a verbal fluency task in patients with schizophrenia. American Journal of Psychiatry, 155(8), 1056–1063.
Desseilles, M., Dang Vu, T., Laureys, S., Peigneux, P., Degueldre, C., Phillips, C., et al. (2006). A prominent role for amygdaloid complexes in the variability in heart rate (VHR) during rapid eye movement (REM) sleep relative to wakefulness. Neuroimage, 32(3), 1008–1015.
Dillon, D. G., & Pizzagalli, D. A. (2007). Inhibition of action, thought, and emotion: A selective neurobiological review. Applied and Preventive Psychology, 12(3), 99–114.
Dolcos, F., Kragel, P., Wang, L., & McCarthy, G. (2006). Role of the inferior frontal cortex in coping with distracting emotions. NeuroReport, 17(15), 1591–1594.
Dolcos, F., & McCarthy, G. (2006). Brain systems mediating cognitive interference by emotional distraction. Journal of Neuroscience, 26(7), 2072–2079.
Franzen, P. L., Buysse, D. J., Dahl, R. E., Thompson, W., & Siegle, G. J. (2009). Sleep deprivation alters pupillary reactivity to emotional stimuli in healthy young adults. Biological Psychology, 80(3), 300–305.
Garavan, H., Ross, T. J., Murphy, K., Roche, R. A. P., & Stein, E. A. (2002). Dissociable executive functions in the dynamic control of behavior: Inhibition, error detection, and correction. Neuroimage, 17(4), 1820–1829.
Germain, A., & Nielsen, T. A. (2003). Sleep pathophysiology in posttraumatic stress disorder and idiopathic nightmare sufferers. Biological Psychiatry, 54(10), 1092–1098.
Gruzelier, J., Seymour, K., Wilson, L., Jolley, A., & Hirsch, S. (1988). Impairments on neuropsychologic tests of temporohippocampal and frontohippocampal functions and word fluency in remitting schizophrenia and affective disorders. Archives of General Psychiatry, 45(7), 623–629.
Hare, T. A., & Casey, B. J. (2005). The neurobiology and development of cognitive and affective control. Cognition, Brain, and Behavior, 9(3), 273–286.
Hartmann, E. (1989). Boundaries of dreams, boundaries of dreamers: Thin and thick boundaries as a new personality measure. Psychiatric Journal of the University of Ottawa, 14(4), 557–560.
Hartmann, E., Elkin, R., & Garg, M. (1991). Personality and dreaming: The dreams of people with very thick or very thin boundaries. Dreaming, 1(4), 311–324.
Hope, D. A., Rapee, R. M., Heimberg, R. G., & Dombeck, M. J. (1990). Representations of the self in social phobia: Vulnerability to social threat. Cognitive Therapy and Research, 14(2), 177–189.
Köteles, F., Szemerszky, R., Freyler, A., & Bárdos, G. (2011). Somatose
nsory amplification as a possible source of subjective symptoms behind modern health worries. Scandinavian Journal of Psychology, 52(2), 174–178.
Krakow, B., Schrader, R., Tandberg, D., Hollifield, M., Koss, M. P., Yau, C. L., et al. (2002). Nightmare frequency in sexual assault survivors with PTSD. Journal of Anxiety Disorders, 16(2), 175–190.
Kunzendorf, R. G., Hartmann, E., Cohen, R., & Cutler, J. (1997). Bizarreness of the dreams and daydreams reported by individuals with thin and thick boundaries. Dreaming, 7(4), 265–271.
Lancee, J., Spoormaker, V. I., & van den Bout, J. (2010). Nightmare frequency is associated with subjective sleep quality but not with psychopathology. Sleep and Biological Rhythms, 8(3), 187–193.
Lane, R. D., Reiman, E. M., Axelrod, B., Yun, L. S., Holmes, A., & Schwartz, G. E. (1998). Neural correlates of levels of emotional awareness: Evidence of an interaction between emotion and attention in the anterior cingulate cortex. Journal of Cognitive Neuroscience, 10(4), 525–535.
Leung, H. C., Skudlarski, P., Gatenby, J. C., Peterson, B. S., & Gore, J. C. (2000). An event-related functional MRI study of the Stroop color word interference task. Cerebral Cortex, 10(6), 552–560.
Levin, R., & Fireman, G. (2002). Nightmare prevalence, nightmare distress, and self-reported psychological disturbance. Sleep, 25(2), 205–212.
Levin, R., Fireman, G., Spendlove, S., & Pope, A. (2011). The relative contribution of affect load and affect distress as predictors of disturbed dreaming. Behavioral Sleep Medicine, 9(3), 173–183.
Levin, R., & Nielsen, T. A. (2007). Disturbed dreaming, posttraumatic stress disorder, and affect distress: A review and neurocognitive model. Psychological Bulletin, 133(3), 482–528.
Levin, R., & Nielsen, T. (2009). Nightmares, bad dreams, and emotion dysregulation: A review and new neurocognitive model of dreaming. Current Directions in Psychological Science, 18(2), 84–88.
Lezak, M. D. (2004). Neuropsychological assessment. USA: Oxford University Press.
MacLeod, C., Mathews, A., & Tata, P. (1986). Attentional bias in emotional disorders. Journal of Abnormal Psychology, 95(1), 15–20.
Maquet, P., Ruby, P., Maudoux, A., Albouy, G., Sterpenich, V., Dang-Vu, T., et al. (2005). Human cognition during REM sleep and the activity profile within frontal and parietal cortices: A reappraisal of functional neuroimaging data. Progress in Brain Research, 150, 219–227.
Mattia, J. I., Heimberg, R. G., & Hope, D. A. (1993). The revised Stroop color-naming task in social phobics. Behaviour Research and Therapy, 31(3), 305–313.
McKenna, F. P., & Sharma, D. (2004). Reversing the Emotional Stroop effect reveals that it is not what it seems: The role of fast and slow components. Journal of Experimental Psychology. Learning, Memory, and Cognition, 30(2), 382–392.
Mellman, T., David, D., Bustamante, V., Torres, R., & Fins, A. (2001). Dreams in the acute aftermath of trauma and their relationship to PTSD. Journal of Traumatic Stress, 14, 241–247.
Milham, M. P., & Banich, M. T. (2005). Anterior cingulate cortex: An fMRI analysis of conflict specificity and functional differentiation. Human Brain Mapping, 25(3), 328–335.
Milham, M. P., Erickson, K. I., Banich, M. T., Kramer, A. F., Webb, A., Wszalek, T., et al. (2002). Attentional control in the aging brain: Insights from an fMRI Study of the Stroop task. Brain and Cognition, 49(3), 277–296.
Moritz, S., Birkner, C., Kloss, M., Jahn, H., Hand, I., Haasen, C., et al. (2002). Executive functioning in obsessive-compulsive disorder, unipolar depression, and schizophrenia. Archives of Clinical Neuropsychology, 17(5), 477–483.
Muzur, A., Pace-Schott, E. F., & Hobson, J. A. (2002). The prefrontal cortex in sleep. Trends in Cognitive Sciences, 6(11), 475–481.
Nielsen, T., & Levin, R. (2007). Nightmares: A new neurocognitive model. Sleep Medicine Reviews, 11(4), 295–310.
Nielsen, T., Paquette, T., Solomonova, E., Lara-Carrasco, J., Colombo, R., & Lanfranchi, P. (2010). Changes in cardiac variability after REM sleep deprivation in recurrent nightmares. Sleep, 33(1), 113.
Nielsen, T. A., Paquette, T., Solomonova, E., Lara-Carrasco, J., Popova, A., & Levrier, K. (2010). REM sleep characteristics of nightmare sufferers before and after REM sleep deprivation. Sleep Medicine, 11(2), 172–179.
Nielsen, T. A., & Zadra, A. L. (2000). Dreaming disorders in principles and practices in sleep medicine. In M. H. Kryger, T. Roth, & W. C. Dement (Eds.), Principles and practices of sleep medicine (3rd ed., pp. 2000). Philadelphia: WB Saunders.
Nielsen, T., & Zadra, A. L. (2010). Idiopathic nightmares and dream disturbances associated with sleep-wake transitions. A. In M. Kryger, T. Roth, & W. C. Dement (Eds.), Principles and practice of sleep medicine (5th ed., pp. 1106–1115). New York: Elsevier.
Nishida, M., Pearsall, J., Buckner, R. L., & Walker, M. P. (2009). REM sleep, prefrontal theta, and the consolidation of human emotional memory. Cerebral Cortex, 19(5), 1158–1166.
Pace-Schott, E. F., Milad, M. R., Orr, S. P., Rauch, S. L., Stickgold, R., & Pitman, R. K. (2009). Sleep promotes generalization of extinction of conditioned fear. Sleep, 32(1), 19–26.
Perret, E. (1974). The left frontal lobe of man and the suppression of habitual responses in verbal categorical behaviour. Neuropsychologia, 12(3), 323–330.
Phaf, R. H., & Kan, K. J. (2007). The automaticity of Emotional Stroop: A metaanalysis. Journal of Behavior Therapy and Experimental Psychiatry, 38(2), 184–199.
Reynolds, D. M., & Jeeves, M. A. (1978). A developmental study of hemisphere specialization for recognition of faces in normal subjects. Cortex, 14(4), 511–520.
Roberts, J., & Lennings, C. J. (2006). Personality, psychopathology and nightmares in young people. Personality and Individual Differences, 41(4), 733–744.
Rueda, M. R., Posner, M. I., & Rothbart, M. K. (2005). The development of executive attention: Contributions to the emergence of self-regulation. Developmental Neuropsychology, 28(2), 573–594.
Schwartz, S., Baldo, J., Graves, R. E., & Brugger, P. (2003). Pervasive influence of semantics in letter and category fluency: A multidimensional approach. Brain and Language, 87(3), 400–411.
Semiz, U. B., Basoglu, C., Ebrinc, S., & Cetin, M. (2008). Nightmare disorder, dream anxiety, and subjective sleep quality in patients with borderline personality disorder. Psychiatry and Clinical Neurosciences, 62(1), 48–55.
Simor, P., Csóka, S., & Bódizs, R. (2010). Nightmares and bad dreams in patients with borderline personality disorder: Fantasy as a coping skill? European Journal of Psychiatry, 24(1), 28–37.
Simor, P., Köteles, F., & Bódizs, R. (2011). Submersion in the experience: The examination of the Tellegen Absorption scale in an undergraduate university sample. Mentálhigiéné és Pszichoszomatika, 12(2), 101–123.
Simor, P., Köteles, F., Bódizs, R., & Bárdos, G. (2009). A questionnaire based study of subjective sleep quality: The psychometric evaluation of the hungarian version of the Groningen sleep quality scale. Mentálhigiéné és Pszichoszomatika, 10(3), 249–261.
Simor, P., Köteles, F., Sándor, P., Petke, Z., & Bódizs, R. (2011). Mindfulness and dream quality: The inverse relationship between mindfulness and negative dream affect. Scandinavian Journal of Psychology, 52(4), 369–375.
Simor, P., Kovács, I., Vargha, A., Csóka, S., Mangel, B., & Bódizs, R. (2009). Nightmares, dream anxiety and psychopathology: the validation of the Hungarian version of the Van Anxiety Scale. Psychiatria Hungarica, 24(6), 428–438.
Sipos, K., Sipos, M., & Spielberger, C. D. (1994). The hungarian version of the state-trait anxiety inventory (STAI). In F. Mérei & F. Szakács
(Eds.), Pszichodiagnosztikai vademecum I/2 (pp. 123–148). Budapest: Nemzeti Tankönyvkiadó.
Spielberger, C., Gorsuch, R., & Lusgene, R. (1970). Manual for the state-trait anxiety inventory. Palo Alto, CA: Consulting Psychologists Press.
Spoormaker, V. I. (2008). A cognitive model of recurrent nightmares. International Journal of Dream Research, 1(1), 15–22.
Spoormaker, V. I., & Montgomery, P. (2008). Disturbed sleep in post-traumatic stress disorder: Secondary symptom or core feature? Sleep Medicine Reviews, 12(3), 169–184.
Spoormaker, Victor. I., Schredl, M., & van den Bout, J. (2006). Nightmares: From anxiety symptom to sleep disorder. Sleep Medicine Reviews, 10(1), 19–31.
Spoormaker, V. I., Sturm, A., Andrade, K. C., Schröter, M. S., Goya-Maldonado, R., Holsboer, F., et al. (2010). The neural correlates and temporal sequence of the relationship between shock exposure, disturbed sleep and impaired consolidation of fear extinction. Journal of Psychiatric Research, 44(16), 1121–1128.
Stroop, J. R. (1992). Studies of interference in serial verbal reactions. Journal of Experimental Psychology: General, 121(1), 15–23.
Tempesta, D., Couyoumdjian, A., Curcio, G., Moroni, F., Marzano, C., De Gennaro, L., et al. (2010). Lack of sleep affects the evaluation of emotional stimuli. Brain Research Bulletin, 82(1–2), 104–108.
Wagner, U., Gais, S., & Born, J. (2001). Emotional memory formation is enhanced across sleep intervals with high amounts of rapid eye movement sleep. Learning and Memory, 8(2), 112–119.
Wagner, U., Hallschmid, M., Rasch, B., & Born, J. (2006). Brief sleep after learning keeps emotional memories alive for years. Biological Psychiatry, 60(7), 788–790.
Walker, M. P. (2009). The role of sleep in cognition and emotion. Annals of the New York Academy of Sciences, 1156(1), 168–197.
Waters, A. M., & Valvoi, J. S. (2009). Attentional bias for emotional faces in paediatric anxiety disorders: An investigation using the Emotional Go/No Go task. Journal of Behavior Therapy and Experimental Psychiatry, 40(2), 306–316.
Whalen, P. J., Bush, G., McNally, R. J., Wilhelm, S., McInerney, S. C., Jenike, M. A., et al. (1998). The emotional counting Stroop paradigm: A functional magnetic resonance imaging probe of the anterior cingulate affective division. Biological Psychiatry, 44(12), 1219–1228.
Wingenfeld, K., Rullkoetter, N., Mensebach, C., Beblo, T., Mertens, M., Kreisel, S., et al. (2009). Neural correlates of the individual Emotional Stroop in borderline personality disorder. Psychoneuroendocrinology, 34(4), 571–586.
Zadra, A., & Donderi, D. C. (2000). Nightmares and bad dreams: Their prevalence and relationship to well-being. Journal of Abnormal Psychology, 109(2), 273–281.
Zysset, S., Müller, K., Lohmann, G., & Von Cramon, D. Y. (2001). Color-word matching Stroop task: Separating interference and response conflict. Neuroimage, 13(1), 29–36.
|

