|

Authors:
PÉTER HALÁSZ1, MARIO TERZANO2, LIBORIO PARRINO2 and RÓBERT BÓDIZS3
1 Neurological Department, National Institute of Psychiatry and Neurology, Budapest, Hungary
2 Department of Neurology, Sleep Disorders Center, University of Parma, Parma, Italy
3 Institute of Behavioural Sciences Budapest, Semmelweis University, Budapest, Hungary
Correspondence: P. Halász, Neurological Department, National Institute of Psychiatry and Neurology,
Hűvösvölgyi út 116, 1021 Budapest, Hungary.
Tel.: +36 1 391 54 36; fax: +36 1 391 54 38; e-mail: halasz@opni.hu
Accepted in revised form 12 December 2003; received 24 September 2003
SUMMARY
The role of arousals in sleep is gaining interest among both basic researchers and clinicians. In the last 20 years increasing evidence shows that arousals are deeply involved in the pathophysiology of sleep disorders. The nature of arousals in sleep is still a matter of debate. According to the conceptual framework of the American Sleep Disorders Association criteria, arousals are a marker of sleep disruption representing a detrimental and harmful feature for sleep. In contrast, our view indicates arousals as elements weaved into the texture of sleep taking part in the regulation of the sleep process. In addition, the concept of micro-arousal (MA) has been extended, incorporating, besides the classical low-voltage fast-rhythm electroencephalographic (EEG) arousals, high-amplitude EEG bursts, be they like delta-like or K-complexes, which reflects a special kind of arousal process, mobilizing parallely antiarousal swings. In physiologic conditions, the slow and fast MA are not randomly scattered but appear structurally distributed within sleep representing state-specific arousal responses. MA preceded by slow waves occurs more frequently across the descending part of sleep cycles and in the first cycles, while the traditional fast type of arousals across the ascending slope of cycles prevails during the last third of sleep. The uniform arousal characteristics of these two types of MAs is supported by the finding that different MAs are associated with an increasing magnitude of vegetative activation ranging hierarchically from the weaker slow EEG types (coupled with mild autonomic activation) to the stronger rapid EEG types (coupled with a vigorous autonomic activation). Finally, it has been ascertained that MA are not isolated events but are basically endowed with a periodic nature expressed in non-rapid eye movement (NREM) sleep by the cyclic alternating pattern (CAP). Understanding the role of arousals and CAP and the relationship between physiologic and pathologic MA can shed light on the adaptive properties of the sleeping brain and provide insight into the pathomechanisms of sleep disturbances. Functional significance of arousal in sleep, and particularly in NREM sleep, is to ensure the reversibility of sleep, without which it would be identical to coma. Arousals may connect the sleeper with the surrounding world maintaining the selection of relevant incoming information and adapting the organism to the dangers and demands of the outer world. In this dynamic perspective, ongoing phasic events carry on the one hand arousal influences and on the other elements of information processing. The other function of arousals is tailoring the more or less stereotyped endogenously determined sleep process driven by chemical influences according to internal and external demands. In this perspective, arousals shape the individual course of night sleep as a variation of the sleep program.
keywords micro-arousal, NREM sleep, K-complex, cyclic alternating pattern (CAP)
SLEEP AND AROUSAL SYSTEM
The major contributions to understanding the nature of sleep were achieved at the beginning of the last century with the rise of experimental physiology, promoted by the Russian physiologist Pavlov (1928) and later with the development of neurophysiology in the western countries (Berger 1930; Bremer 1935; Dempsey et al. 1941; Moruzzi 1972). What emerged from these pioneering studies was the sequence of events that rules the cyclic alternation between sleep and wakefulness. The sleep-promoting systems are concentrated in the medial part of the brainstem, dorsal reticular substance of the medulla, anterior hypothalamus and basal forebrain. The wake-promoting areas are mainly concentrated in the pontine and midbrain tegment, the posterior hypothalamus, and the basal forebrain cholinergic neurons. These structures are included in the arousal and activating systems during sleep. Compared with wakefulness, sleep is subjectively perceived as a reduced responsiveness to environmental stimuli induced by a selective closure to the inputs arriving from the external world (Steriade 2000a). The filter that gates the flux of information from the peripheral receptors to the cortex is situated in the thalamocortical connections where the incoming signals are blocked or attenuated via synaptic inhibition. This mechanism modulates the susceptibility of the cerebral cortex to all the activating stimuli. In particular, the generators of cortical electrical activity are modified during sleep and shift from the production of low-amplitude high-frequency electroencephalographic (EEG) activity (LAHF mode) typical expression of the massive activation of the cortical cells, to the production of high- amplitude low-frequency EEG activity (HALF mode) indicating a widespread synchronization of the cortical cells (Steriade and Llinas 1988; Steriade and McCarley 1990; Steriade et al. 1990). Rapid eye movement (REM) sleep, which appears later in the night, is characterized by the simultaneous occurrence of LAHF EEG activity, absence of muscle tone and recurrent rapid eye movements. If EEG slowing is considered as the expression of a reduced cortical activation typical of sleep, the presence of REM sleep is undoubtedly a paradoxical condition, which indicates that sleep does not exclude states of transient cerebral and cortical activation. The alternation between non-REM and REM sleep is the outcome of a balanced action based on the cyclic function of brainstem structures (McCarley and Hobson 1975).
ACTIVATED STATES OF THE BRAIN
Attention to the functions and importance of activated brain states was first raised by
Moruzzi and Magoun (1949) who demonstrated an ‘activation’ process in the changes of EEG waves and verified their brainstem origin. The discovery and localization of brainstem reticular arousal system (RAS) was made by lesion experiments and by electrical stimulation of the brainstem reticular core (Hobson 1978). The EEG effect of arousal was called as ‘desynchronization’, but the coincidence of desynchronization with the concept of arousal has created severe limitations to the future development of the field. Although slow synchronization thalamic devices are really decoupled and the EEG activity is flattened, a fast (30–40 Hz) EEG pattern synchronization appears in the cortical and thalamic networks (Steriade 1995). Accordingly, the term ‘desynchronization’ should be considered as a rapid shift from HALF, typical of sleep, to LAHF, typical of wakefulness.
The pathways and the chemical codes for this function have been explored in detail. Activation in the wake state and in REM is prolonged in duration (expressed by an EEG tonic pattern) and is associated with a sustained depolarization and tonic firing in thalamocortical neurons, enhancing the probability of thalamic responses to incoming volleys. Excitation of RAS neurons enhances cortical-evoked potentials (Bremer and Stoupel (1959). The arousal effect can be evoked by stimulation from the mesopontine cholinergic nuclei (Jones and Webster 1988; Montplaisir 1975) and by stimulation of the locus coeruleus (Steriade and McCarley 1990) setting into action another noradrenergic activation pathway. However, the vast majority of those reticular neurons, which take part in the classical arousal effect, are glutamatergic (Steriade 1995). Now it is clear that there are several arousal systems in the brainstem working in a more differentiated way during the wake state and REM sleep than it was imagined. The most important neurochemical difference between the two activated brain states is that during REM the monoaminergic (noradrenergic and serotoninergic) neurons are silent (Hobson et al. 1975; McGinty and Harper 1976)
Functional neuroimaging studies proved several differences between arousal in wakefulness and REM sleep, characterized by a specific distribution of blood flow and glucose utilization patterns, which delineates different sites of cerebral-activated function. The brain areas where REM sleep neural activity is higher than in wakefulness are the anterior cingulate cortex (Braun et al. 1997; Buchsbaum et al. 1989; Nofzinger et al. 1997), the amygdala and the limbic–paralimbic regions (Braun et al. 1997; Nofzinger et al. 1997), and the associative visual areas (Braun et al. 1997, 1998; Madsen et al. 1991). In the subcortical structures the pons is significantly more active during REM sleep than during wakefulness (Braun et al. 1997). Conversely, neural activity in prefrontal association areas (Braun et al. 1997; Madsen et al. 1991) and in the inferior parietal association cortex (Braun et al. 1997) is lower during REM sleep than during wakefulness. The co-activation of limbic–paralimbic regions and higher order visual processing areas together with the absence of frontal activation was interpreted as a closed-loop operation between the limbic and the visual system, which could be the neurobiologic basis of dream mentation (Braun et al. 1998). An REM sleep-specific activation of the amygdala was found compared with the unified data of wakefulness and NREM sleep (Maquet et al. 1996), as well as a positive correlation between amygdala activity and temporal lobe activation during REM but not during wakefulness (Maquet and Phillips 1998). The amygdala is a significant modulator of brain activation during REM sleep but not during wakefulness. In general, subcortical and midline structures are more active during REM sleep than during wakefulness. Once again functional neuroimaging studies delineated significant differences between wakefulness and REM sleep-related arousal.
Arousal from REM sleep deserves more studies. Cantero and Atienza (2000) suggest that some burst of alpha activity may indicate a state specific micro-arousal (MA) in REM sleep. The same idea was incorporated in the extension of CAP concept to REM sleep by Terzano et al. (1985) in their first description of the pattern. The transitory occurrence of alpha activity in REM sleep was in the same time used to explain the phenomena of ‘lucid dreams’ (Ogilvie et al. 1982). According to these results and interpretation alpha bursts in REM sleep may represent a transitory gate toward wakefulness.
INTERACTION BETWEEN THE SLEEP SYSTEM AND THE AROUSAL SYSTEM
Sleep regarded as a state condition of brain-body rest conflicts with the principle that life requires permanent activity of essential functions. Because the brain is the organ that warrants the continuity of these functions, the issue of brain rest during sleep is misleading. The brain is permanently active and is able to control the autonomic, metabolic and hormonal changes that take place within the body and simultaneously determine the behavioral responses to the external stimuli. Although sleep is characterized by decreased conscious perception, these tasks are accomplished nevertheless during sleep through a gradual activation of the brain or through a partial activation confined to some cerebral areas (Ujszászi and Halász 1988). Such activation is stimulated by the arousal system.
During NREM sleep the systems responsible for activated states are actively inhibited (Szymusiak et al. 2001) and the reciprocal antagonistic relationship between sleep and arousal system preserves on the one hand life against danger and on the other support the natural evolution of sleep (Saper et al. 2001).
The sleep promoting system and the arousal-promoting system are the two pillars that bridge the internal process of sleep to the external world. In this way, the sleeping brain not only regulates the reactions but also assimilates in its functions the incoming information. From a functional point of view, sleep modulates and is modulated by this series of interactions.
AROUSAL FROM SLEEP
The term ‘arousal’ conventionally indicates a temporary intrusion of wakefulness into sleep (Atlas Task Force 1992), or at least a sudden transient elevation of the vigilance level due to arousal stimuli or to spontaneous vigilance level oscillations. When arousal interrupts sleep in a definitive, not reversible way, we speak about awakening. Arousal is a ‘relative concept’. It is not possible to understand arousal without the related vigilance state. Aroused state and sleep are two different sides of vigilance; we cannot define them without each other. To speak about arousal in sleep may sound controversial. There are however strong evidences that one of the essential features of sleep is the arousability and presence of abundant arousals.
The concept of arousal has a long history, which is closely connected with the development of concepts about the neurophysiology of sleep and wakefulness. The criteria and measure of arousal are controversial issues; hence, arousal has several definitions (Halász et al. 1979; Lofaso et al. 1998; Martin et al. 1997a; Rees et al. 1995; Schieber et al. 1971; Terzano et al. 1985) and several EEG, behavioral and autonomic aspects. The EEG resultant of arousal has massive impact on the evolution of the sleep profile. Behavioral and autonomic concomitants of arousal may or may not be present at the same time. When they are present, they can be graduated in intensity, while presence or absence of the single components of the arousal constellation depends on the involvement of the specific cerebral compartments. The questions are: which constellations or single signs are sufficient to be accepted as arousal markers? How should we consider specific EEG phasic events characterized by slow waves but still endowed with activating properties? How should we classify the somato-vegetative phenomena not associated with any detectable EEG modification?
To ad
dress these questions available data on the relationship between different aspects of arousal and the underlying neural mechanisms need to be reviewed. In the present paper, we will focus our attention on the nature of arousal in sleep in the broader context of dynamical changes during sleep. We will provide evidence in favor of a hierarchical continuum of arousal phenomena showing multiple features and multiple components. These varieties depend on the state of the sleeper and the source and strength of the arousal generator, but all sharing a homogeneous neurophysiologic background.
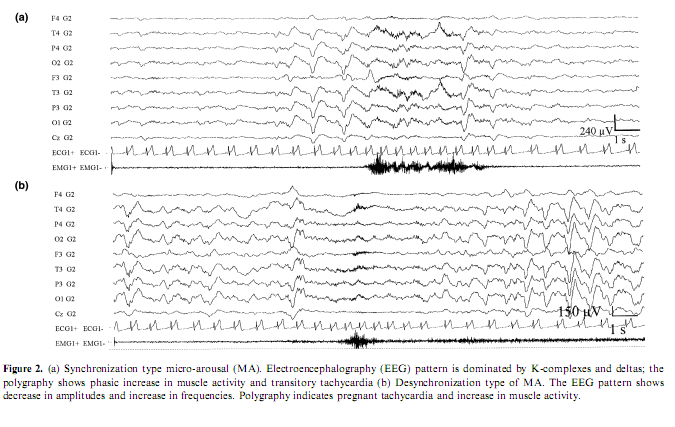
The overview and reconsideration of this topic seems to be appropriate for several reasons. In the last years a considerable pool of data has been accumulated from different sources with heterogeneous approaches and views about the phenomenology of arousals in sleep both under physiologic and pathologic circumstances. There is an endeavor to categorize and standardize arousals from sleep; however there are several contradictions and controversial views around this issue. The American Sleep Disorders Association (ASDA) produced a consensus report (Atlas Task Force 1992) on the criteria of arousals in sleep in the early 1990s. Arousal is defined as a rapid modification in EEG frequency, which can include theta and alpha activity, and/or frequencies higher than 16 Hz but not spindles. It can be accompanied by an increase of electromyographic activity, of cardiac frequency or by body movements. An arousal must be preceded by at least 10 s of continuous sleep. According to these rules, slow EEG features such as K-complexes and transient delta activities were not scored as arousals unless these patterns were associated with an EEG frequency shift toward theta, alpha or beta rhythms. For these reasons, the proposed scoring system for arousals was strongly criticized by other research groups who have been engaged in the last 30 years with the study of the microstructure of sleep (Terzano et al. 1991a). The conceptual basis of the ASDA criteria is that arousal is a marker of sleep disruption, a detrimental and harmful thing, while our study indicates arousals as elements weaved into the texture of sleep taking part in the regulation of the sleep process (Terzano and Parrino 1993a). Beyond different conceptual approaches the essence of the controversy is to include or exclude into the concept of arousal those evoked or spontaneous elements of the EEG which are characterized by high-voltage slow rhythms and/or K-complexes and spindles instead of the traditional shift toward rapid rhythms and voltage decrement, associated with the same kind of behavioral and autonomic changes typically accepted as arousal signs. From a practical point of view it is questionable why those elements which are not signs of cortical activation, although they are proved to be reactive EEG patterns, are disregarded as arousals or as a prearousal activation although most times they precede the EEG arousal signs (De Carli et al. in press; Halász 1993; Halász and Ujszászi 1991; Roth et al. 1956). In effect, the ASDA criteria neglect to consider the abrupt appearance of slow sleep elements (K-complexes, delta bursts) as arousals even when they are associated with somato-vegetative modifications identical to those observed during arousals (Ferini-Strambi et al. 2000; Ferri et al. 2000; Sforza et al. 2000a). To overcome the general assumption that only arousals are markers of cortical activation the coding system of cyclic alternating pattern (CAP) identified different EEG features endowed with activating properties and coalesced into a common ‘brain beat’ (Terzano et al. 1985). In the CAP framework, arousals are viewed as complex phenomena involving not only cortical areas but also other brain centers and peripheral neural components (Fig. 1). These components are involved with different latencies and intensities but are nevertheless transformed into a unitary phenomenon by the reciprocal interneural connections (Moruzzi 1972). The activating phenomena occurring within the somato-vegetative systems do not always correspond to a cortical activation, as the arousal definition seems to suggest. The outcome of stimulation can also be a mild cortical activation expressed as a mixed slow-rapid EEG pattern (as for subtypes A2 of CAP) or can evoke a protective reaction of the sleeping brain (as for subtypes A1 of CAP). In the latter case, arousal is aborted and the response characterized by slow EEG pattern typical of NREM sleep is more an anti-arousal phenomenon that protects the continuity of sleep instead of fragmenting it (Hirshkowitz 2002). From these considerations, it derives that if all arousals are signs of activation not all the sleep EEG patterns related to activation correspond to the conventional definition of arousals (Atlas Task Force 1992). Therefore, the activation response during sleep is not limited to a single pattern but is part of a continuous spectrum including EEG- synchronized features, EEG-desynchronized features or a combination of both (Fig. 2).
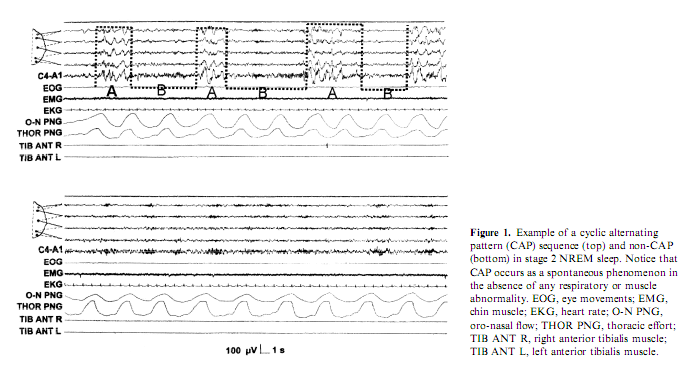
WHAT IS A MICRO-AROUSAL AND HOW IS IT RELATED TO THE COURSE OF SLEEP?
The term MA was first systematically used to designate those phasic EEG events which were not associated with awakenings regardless of their desynchronizational or synchronizational (sleep response-like) morphology and regardless of their connection with autonomic or some sort of behavioral arousal (Halász et al. 1979). Concerning the traditional desynchronization type morphology the phenomenon was described entirely by the early work of Schieber et al. (1971) named at that time as ‘phases d’activation transitoire’ (PAT). The criteria for MA in NREM sleep given by Schieber et al. (1971) were the following: increase in EEG frequencies in conjunction with decrease of amplitudes, disappearance of delta waves and spindles, transitory enhancement of muscle tone or phasic appearance of groups of muscle potentials, movements of the limbs or changes in body posture, transitory rise in heart rate. In REM sleep the criteria for MAs were temporary disappearance of eye movements and appearance of alpha activities. The duration of these changes varied from some seconds to more than 10 s. Temporary ‘activation’ is followed by ‘deactivation’ leading to a bi-phasic character of the phenomenon. The term was modified by several workers in the last years and used in the context of physiologic and pathologic studies, with more or less the same meaning and criteria (Quattrocchi et al. 2000; Sforza et al. 2002). It is however surprising how in the following studies the phenomenon of postarousal deactivation has been neglected, while it has been successively recovered and amplified in the description of the so called phase B of CAP (Terzano and Parrino 1993b; Terzano et al. 1985).
The occurrence of PAT like MAs is inversely proportional to the depth of sleep, occurring more frequently in superficial than in deep sleep with the highest incidence during REM sleep and stage 1, appearing the least frequently during stage 3 and 4. The distribution of MA is not homogeneous across the sleep cycles. MA are more frequent during the ascending slopes of the cycles compared with the descending slopes, and their frequency increases from evening to morning (Ferrillo et al. 1997; Halász 1982; Schieber et al. 1971; Terzano and Parrino 2000; Terzano et al. 2000).
THE CONCEPT OF CORTICAL, SUBCORTICAL AND AUTONOMIC AROUSAL
Those clear-cut arousals, which have enough activating strength to change the level of vigilance on a macro-scale, are characterized by a threefold phenomenology involving simultaneously EEG, behavioral and autonomic compartments. The conventional definition of arousal includes a cluster of physiologic manifestations expressed by an activation of electrocorticographic rhythms, an increase of blood pressure and muscle tone and a variation of
heart rate. Arousal has been considered as an essential element for restoration of homeostasis during respiratory and cardiovascular failure during sleep providing an excitation drive to vital processes. Arousal, by definition, means cortical activation. However, somatosensory and auditory stimulation during sleep may result in cardiac, respiratory and somatic modifications without overt EEG activation (Carley et al. 1997; Winkelman 1999). This observation implies that there is a range of partial arousal responses with EEG manifestations different from classical arousals and even without any EEG response. The different arousal responses rely on the different combinations of the central and peripheral components, on the intensity scale of their manifestation, and on the morphologic variations of the cortical reactions. The spectrum of combination of the three compartments in which arousal can appear is a matter of debate. Any behavioral expression, which occurs associated with low-voltage fast-EEG activities, is classified as a ‘behavioral arousal’ (Moruzzi and Magoun 1949). Similar features are shown by ‘movement arousals’ described as any increase in electromyographic activity that is accompanied by a change in any other EEG channel (Rechtschaffen and Kales 1968). When the EEG compartment is involved by transient desynchronization patterns, regardless of the participation of the autonomic system or behavioral components, it was held as ‘cortical arousal’ (Atlas Task Force 1992). When there is evidence of vegetative or behavioral activation associated with an EEG pattern different from conventional arousal the event was defined as ‘subcortical arousal’ (McNamara et al. 2002; Rees et al. 1995). When an autonomic activation appears isolated or in conjunction with a respiratory event, but without any concomitant EEG sign, it is commonly defined as an ‘autonomic arousal’ (Martin et al. 1997b; Pitson and Stradling 1998). There is an autonomic ‘overarousal’ compared with the periods of arousal from continuous awake state during periods of awakening from NREM sleep (Horner et al. 1997), that also represents a certain kind of quantitative decoupling between the autonomic and other components of arousal.
The dichotomy of EEG/autonomic arousal versus movement/behavioral arousal does not need much explanation; hence placed on a gradual scale the latter represents obviously a stronger activation. This is supported by the fact that movement and behavioral arousals without either EEG or autonomic concomitants do not exist. In contrast, there is evidence that an arousal from sleep is associated with heart rate acceleration and blood pressure increase even in the absence of any behavioral or somatomotor activity (Trinder et al. 2001, 2003).
The hierarchical relationship between the compartments becomes clearer by taking into consideration the time relationships between the components (Kato et al. 2003; Riva et al. 2002). As the autonomic component may precede the EEG component (Bonnet and Arand; 1997a), the cortical compartment could not be considered as the univocal source of autonomic activation. As both the EEG and vegetative reactions can appear decoupled, these two kinds of arousal manifestations may have separated and independent physiologic substrates activated simultaneously by the same input. The temporal overlap between cortical, somato-motor and vegetative events within the same arousal episode does not necessarily imply synchrony and the order of activation of the single compartments can vary in the different physiologic or pathologic circumstances (Karadeniz et al. 2000). In arousal phenomena during sleep there is no mandatory chronologic and etiologic subordination. The phenomenon takes place within interactive loops in which the cerebral cortex can be the starting or the ending point but anyway a source of control. The origin of arousal should be defined by the subsystem primarily activated or perturbed. Arousal can be generated directly by the cortex under the impulse of the physiologic evolution of sleep or in response to a sensorial perturbation, such as respiratory interruption, noisy environment, alteration of blood pressure or heart rate, or a movement disorder. In any case, it is the involvement of the brain that makes arousal a unitary phenomenon in which activation is modulated through a hierarchy of responses ranging from the generalized activation of all subsystems to the controlled attenuation of arousal-inducing activation (Black et al. 2000).
ELECTRODERMOGRAPHIC ACTIVITY AS AN INDICATOR OF AROUSAL LEVEL DURING SLEEP
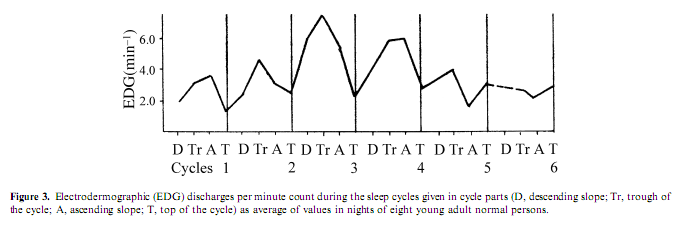
Electrodermographic (EDG) activity, measured by the spontaneous fluctuations of skin conductance, is a rarely investigated aspect of autonomic activity during sleep; the association of EDG discharges with arousal was proved by several studies (Jung 1954). Therefore the consequent finding of several authors about a ‘storming effect’ in EDG activity during slow wave sleep was astonishing. (Broughton et al. 1965; Freixa et al. 1983; Halász et al. 1979; Johnson and Lubin 1966; Jung 1954; Lester et al. 1967; Liguori et al. 2000; McDonald et al. 1976). Investigating EDG activity in different experimental conditions, including psychostimulant drug administration, sleep deprivation, and random sensory stimulation, we found that the frequency of EDG discharges was under the influence of sleep depth and cycle order. The highest activity occurred during the second and third sleep cycle and during slow wave sleep, while in REM sleep the occurrence rate was similar to wakefulness (Halász et al. 1980). The activity was highest under the influence of a psychostimulant and lowest during the night after sleep deprivation, while baseline and placebo nights showed values in between. During the sleep cycles the EDG activity increased parallel with the deepening of sleep across the descending slope (DS), which is the first part of the NREM component of the sleep cycle and is characterized by the transition from light (stages 1 and 2) to deep (stages 3 and4) sleep, persisted at a high level during the cycle trough, which is the period of persistent deep sleep, decreased abruptly after the cycle turn, across the ascending slope (AS), which is the period of transition from deep to light NREM sleep and became lowest during REM sleep (Fig. 3). Some authors (Broughton et al. 1965; Johnson and Lubin 1966; Jung 1954) have assumed that the increase of EDG activity in NREM sleep is due to the release of the RAS from cortical inhibition. But in our study the amount of slow wave sleep and the EDG activity did not run parallel, and even during deep sleep the EDG activity preserved its dependence from the activation level. Lester et al. (1967) showed that previous day stress effect increased the level of EDG activity during the next night and anxiousness as a personality dimension also enhanced the level of slow wave sleep EDG storming. In contrast to the assumption of the static reciprocal inhibition between sleep promoting and arousal influences (Saper et al. 2001) we interpreted the association between the deepening tendency of sleep across cycles and the increasing occurrence of EDG discharges as the sign of an arousal process promoting the cycle turning and the ensue of the AS. This assumption is supported by the findings of Curzi-Dascalova and Dreyfus-Brisac (1976) and Curzi- Dascalova et al. (1970) who could not find EDG storming during quiet sleep of neonates, until the development of sleep spindles. That means the development of EDG storming is parallel in infants with the development of thalamic slow wave sleep circuitry.
AROUSALS PRECEDED BY SYNCHRONIZATION (SLOW WAVES, K-COMPLEXES) EEG CHANGES
Overall, arousal phenomena are characterized by an extensive variability not only due to different degrees of behavioral or autonomic participation but also by the wide variability of EEG features. The recognition of this kind of variability has a long story and has required
a development of views. Evidences come from two different sources. One of them is the research on K-complexes, which are distinctive elements of NREM sleep, especially of stages 2 and3, endowed with controversial properties. Some authors (Roth et al. 1956; Sassin and Johnson 1968) consider these features as partial forms of arousal, while others (Nicholas et al. 2002; Waquier et al. 1995) indicate these elements as sleep-protective events. According to a combinatory viewpoint, these events are endowed with both activating and preserving properties (Halász et al. 1985a). Recently Bastuji et al. (1995) developed the ‘forced awakening’ method. In this paradigm subjects were questioned about quantitative and qualitative aspects of stimulus recall evoked by ‘oddball’ type stimuli in parallel with recording of the evoked cortical responses, after being aroused by the stimuli from naps. In subjects whose quality of recall was excellent, P300 waves were indistinguishable from those obtained before sleep. When P300 was found attenuated, delayed and desynchronized, recall was quantitatively degraded and P300 was concomitant to or replaced by sleep negativities (varieties of late negative components being part of the K-complex) in subjects in whom stimulus recall was severely degraded or absent (Garcia-Larrea et al. 2002). They concluded that K-complex analog sleep negativities have two aspects being, on the one hand, arousal driven and, on the other, ‘erasers’ preventing accurate memory encoding and retrieval of the stimulus, promoting consequently sleep. The same ‘Janus faced’ nature of K-complexes were stressed by us previously (Halász 1982) and the possible functional importance of this aspect will be treated later. An increase in the amplitude of the K-complex N350-550 and P900 components after sleep deprivation has been described recently (Peszka and Harsh 2002). This again shows clearly that K-complex characteristics are very close to those of delta sleep (Nicholas et al. 2002).
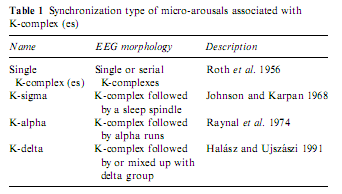
The first studies of K-complexes showed that these graphoelements, which are held to be the building stones of slow wave sleep (De Gennaro et al. 2000), are elicitable by all modalities of sensory stimuli (Bastien and Campbell 1992; Niiyama et al. 1995; Roth et al. 1956; Sallinen et al. 1994) and are accompanied by autonomic discharges identical to those seen for arousals (Ackner and Pampiglione 1957; Fruhstorfer et al. 1971; Guilleminault and Stoohs 1995; Hornyak et al. 1991; Johnson and Karpan 1968; Sassin and Johnson 1968; Sforza et al. 2000a, 2002; Takigawa et al. 1980). Later it was shown that K-complexes rarely remain single events but are accompanied by other rhythms such as K-delta, K-alpha and K-spindle according to the nature of the associated rhythm (Halász and Ujszászi 1991; MacFarlane et al. 1996; Raynal et al. 1974). These complex events beginning with K-complexes are frequently followed by long-lasting changes in the ongoing EEG, associated with distinct autonomic modifications (Table 1).
Accordingly they could be considered as a ‘synchronization type’ of MA (Halász et al. 1985a). We report that arousals proceeded by slow waves and K-complexes have a different distribution across the sleep stages compared with the ‘desynchronization type’ of MA. The former showed the greatest occurrence rate during slow wave sleep, being most frequent in stage 2 (Halász et al. 1985b). Sforza et al. (2000a) scored 5820 events during the night sleep of 21 young adult volunteers. Thirty-two percent of events were scored as arousals and in 40% they were preceded by isolated K-complexes. PAT type arousals represented 23% of the total arousals whereas delta and K-complex bursts tended to occur mostly during the first two sleep cycles. Other types of MA and clear-cut PAT occurred during all sleep cycles with a greater density in light and REM sleep. In an analysis carried out on 40 healthy subjects, Boselli et al. (1998) ascertained that the number of arousals during NREM sleep increase with age. In the same sample, 87% of arousals were preceded by a K-complex or a delta activity and showed a positive correlation with stages 1 and2 (Terzano et al. 2002). Delta and K-bursts were concentrated in the first three sleep cycles and presented a divergent behavior compared with ASDA arousals (Parrino et al. 2001).
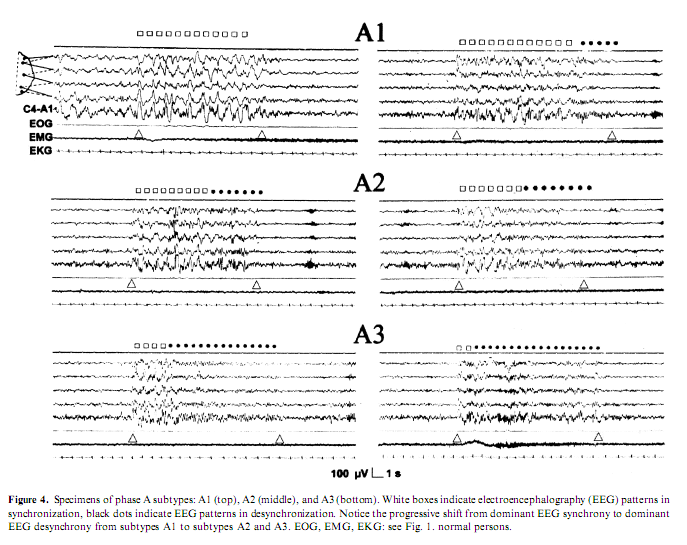
Another line of evidence for different types of arousals came from the discovery of CAP (Terzano et al. 1985). It was shown that the CAP A-phase behaves like the synchronization arousals, can be elicited by sensory stimuli and is associated with clearly detectable autonomic discharges. Later the Parma school differentiated within the CAP A-phase three subtypes (Fig. 4). In subtype A1, EEG synchrony is the predominant activity. If present, EEG desynchrony occupies 50%of phase A occupied by EEG desynchrony. Subtype A3 is coupled with a remarkable activation of muscle tone and/or autonomic activities (Terzano et al. 2001). The distribution of the different phase A subtypes has been proved to be different across the sleep cycles. Subtypes A1 occur most frequently in the first cycles of sleep and during the DS of the cycles, while subtypes A2 and A3 subtypes are more frequent during the later part of sleep and in the AS of the cycles (Terzano and Parrino 2000). Therefore we can identify the A1 subtype with the ‘synchronization arousal’ of Halász et al. (1985a), and the A3 subtype with the original PAT of the Strasbourg School (Schieber et al. 1971), while the A2 subtype is a mixed one between the two (Parrino et al. 2001). Now it is clear that the most important factors that determine the variable EEG morphology of arousals appearing in NREM sleep are the linkage with stages and the position of the given arousals within the course of sleep.
Other important factors are the nature and the intensity of the stimulus originating the arousal.
THE INFLUENCE OF SENSORY STIMULATION ON THE FORMATION OF BOTH TYPES OF AROUSALS
Sensory stimuli can evoke EEG arousals with or without behavioral and autonomic changes and their phenomenology are exactly the same as experienced in the so-called spontaneous arousals. Ehrhart and Muzet (1974) showed that PAT could be elicited by sensory stimuli. Stimulation decreased the number of the spontaneous PATs; however, the total (spontaneous and evoked) number of PATs was similar to the number of the spontaneous PATs without stimulation. Under the influence of a psychostimulant drug the frequency of MA slightly increased and the difference in the distribution between the DS and AS of the cycles disappeared. Sensory stimulation did not affect the average frequency of MA, but occurrence during deep sleep increased and the difference between the AS and DS decreased in a relevant way due to the increased frequency during the DS. Elicitability by sensory stimulation was best in superficial sleep stages and worst in deep sleep that means it went parallel with rates of spontaneous occurrence (Halász et al. 1979).
Concentrating purely on K-complexes regardless of the other associated rhythms Halász (1982) found a significant increase in the number of K-complexes under the influence of continuous random sensory stimulation during stage 2 of NREM. The elicitability of K-complexes was higher during the AS of cycles (where the spontaneous occurrence rate was also higher) compared with the DS. Under stimulation the number of spontaneous K-complexes decreased, but the total number (spontaneous and evoked) of K-complexes increased.
Using sound stimulation with 90-dB tones at 625 Hz, 1/1 to 1/5 min rate delivered by headphones, Levine et al. (1987) found that the number of ‘natural arousals’ decreased during nights with frequent (1/1 min) stimulation resulting into abundant evoked arousals. The polygraphic characteristics of these arousals were not sho
wn, but similar findings were described (Nicholas et al. 2002).
STATE-SPECIFIC REACTIVITY IN SLEEP
Here we arrive at a more dynamic view in the understanding of the nature of arousal in sleep. We must introduce an otherwise well-known biologic concept, namely the ‘state-specific reactivity’. In a certain biologic state the reactivity of the organism to stimulation is determined by the given state in which the input arrives. It is well known that sensory reactivity is different in REM and NREM sleep. However, the change in reactivity within NREM depending on whether the stimulus arrives during the descending or ascending part of the sleep cycle needs further elaboration. First of all we should know more about the phenomenological and physiologic differences of the two slopes. There are not many studies on this topic. The first mention about the asymmetry of the DS and AS of sleep cycles was made by Williams et al. (1964, 1966) and confirmed by automatic analysis of sleep signals (Dijk et al. 1990; Merica and Fortune 1997). On the DS the deepening of sleep occurs more slowly and gradually while the duration of AS is shorter, the changes are more abrupt, and sometimes a stage could be skipped. Sinha et al. (1972) claimed to forecast the times of morning awakening by studying the tendency of AS tangential across the hypnograms. Halász (1982) measured and compared the duration of DS and AS slopes and the number and sequence of phase shifts in healthy volunteers. The net result of this study was that sleep cycles – at least in the first part of sleep where this phenomenon was possible to investigate – are asymmetric: the DS is longer and sleep stages shift gradually, the AS proved to be shorter, and changes less gradual. In other words, the AS is steeper and 30–50% shorter compared with the DS (Terzano et al. 2000).
The differences between the frequency and morphologic features of arousals are in harmony with the asymmetric dynamics of the two slopes of sleep cycles. Across the DS and most prominently in the first cycles arousals are less frequent, show slower EEG activities, are associated with only mild autonomic perturbations, while across the AS arousals are more frequent, and the EEG morphology and the concomitant autonomic changes fulfill better the conventional arousal expectations (Terzano et al. 2000). These polysomnographic findings were confirmed by computerized analysis that showed an increase of very fast rhythms in the final part of the sleep cycle, when NREM sleep precedes the onset of REM sleep and a sharp reduction of these rapid rhythms at the beginning of NREM sleep in the following sleep cycle (Ferri et al. 2001).
On the basis of these findings we may speculate that the differences in arousals might reflect an intimate relationship between state responsivity and the tendencies of state shifts according to the sleep profile (Table 2).
State determines sensory responsivity and the sensorial stimulation – both in experimental and spontaneous situations – may contribute to the state shifts. Sleep state shifts are determined basically by chemical changes governed by brainstem influences. During the NREM–REM cyclicity there are slowly moving tonic changes underlain by the cyclic alternation of brainstem aminergic and cholinergic influences. Besides the involvement of chemical changes, the alternation of DS and AS during the NREM component of the sleep cycle can be influenced by the appearance of arousals, which also reflect the influence of external factors on the sleep process. The dynamics experienced in arousals suggest that sensory stimuli may participate in the determination of the sleep profile and co-operate in shaping the course of sleep cycles. We can formulate this kind of double, ‘tonic’ and ‘phasic’ regulation, in which the effect of ‘phasic’ arousals are tuned by the background ‘tonic’ chemical influences, and at the same time ‘phasic’ stimulation contributes to changes in ‘tonic’ influences.
It is clear that the DS and AS portions of the sleep cycles represent two different substates. During the DS slope sleep- promoting influences are overwhelming and the arousal system is more inhibited compared with the AS (Evans 1993). During this tonic sleep dominance the thalamo-cortical system works in the bursting mode and the influence of brainstem arousal systems are tonically repressed (Steriade and Llinas 1988). Accordingly, in the DS phasic arousal events are rare and they are often mixed with sleep-like responses. Here we do not observe a complete breakdown of NREM bursting mode in the thalamocortical network but it seems as if the distinct subsystems are in conflict and influence each other reciprocally throughout the arousal response (De Carli et al. in press; Steriade and McCarley 1990). The slow EEG pattern elicited by the arousing stimulus, which characterizes the first part of the response, seems to prevent or attenuate the depolarizing influence of cholinergic innervations of thalamic relay cells. The outcome is a balance between anti-arousal and arousal responses (Hirshkowitz 2002). There are two experimental studies investigating the effect of arousal stimuli during the bursting mode. Szymusiak et al. (1996) applied rostral midbrain monophasic electric stimulation with 0.2 ms duration and 100–800 lA and registered a state-dependent effect on thalamic single unit activity. While stimulation during wake state and REM sleep evoked a short-latency action potential, during NREM sleep stimulation commonly evoked a high frequency burst of action potentials followed by a period of suppressed discharge. In the majority of neurons a second rebound burst of action potentials followed the period of discharge suppression. The average interval between the initial and rebound burst was similar to the interburst interval recorded in the same cells during spontaneous EEG spindles. The authors conclude that stimulation of the reticular formation evoked rhythmic discharges dependent upon the presence of thalamo-cortical synchronization. Mariotti et al. (1989) showed that in the nucleus VPL of the thalamus the response to peripheral physiologic stimulation during NREM sleep shows three main components: a very brief and scanty excitatory response, followed by a long period of discharge suppression and by excitatory rebound. The landmark of arousal is a strong increase of the excitatory response and a marked reduction of the inhibitory phase, eventually with disappearance of the rebound. Another possibility to understand the slow wave response to sensory stimulation would be that the sensory input which arrives to the cortical level meets the slow (<1 Hz) depolarizing oscillation, nowadays identified as the K-complex generator (Amzica and Steriade 1997), in the phase of fast (30–40 Hz) rhythm which allows MA in slow wave sleep without long-lasting interruption of the inhibitory rebound sequences in the thalamocortical network.
After the cycle turns to the AS the dominance of NREM sleep decreases. The neurochemical background of this weakening influence in the second part of the cycle could be the combined result of a decreased amount of NREM sleep supporting monoamines and an increasing antagonizing REM promoting cholinergic influence, according to the ‘reciprocal-interaction’ hypothesis of Hobson et al. (1975). This decrease in NREM sleep-promoting influence results in an increase in the phasic arousal activity which now has a better cortical arousal effect reflected in the more arousal-like type EEG, behavioral and autonomic activity. This increase in arousal activity also results in more intensive arousals conjoined from time to time not only with transitory EEG reactions but also with stage shifts. The gradual weakening of NREM sleep and the increasing dominance of REM forces can explain the asymmetric conformation of the sleep cycle with a smoother DS and a steeper AS.
THE HIERARCHY OF AROU
SALS – THE CONCEPT OF A CONTINUUM
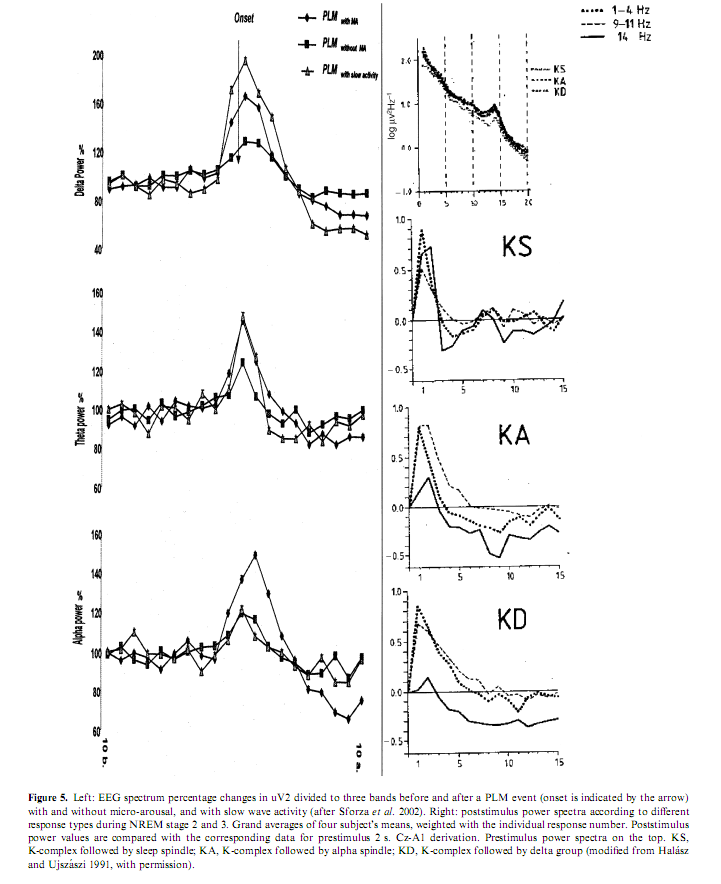
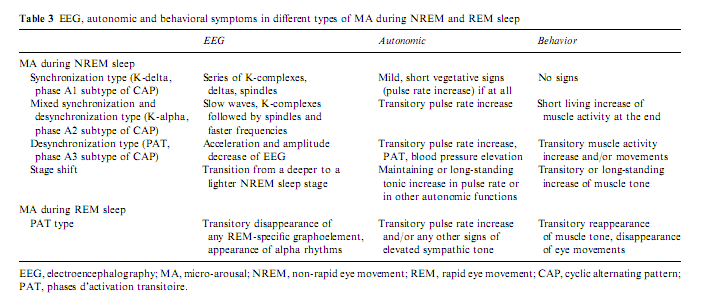
As previously illustrated, arousals are variable according to the different combinations of their EEG, behavioral and autonomic activities. The phenomenology of arousals is influenced not only by stages and sleep depth but also by the tendency of the sleep process. If arousals really reflect the same physiologic process of activation they should behave in a stereotyped way regardless of the involvement of the different peripheral compartments. Sforza et al. (2002) showed a stereotyped rising and falling in the delta, theta, and alpha power associated with periodic leg movements regardless of the presence or absence of EEG arousals and regardless of the absence or presence of slow EEG waves in arousals (Fig. 5). Similar spectral changes were described by Halász and Ujszászi (1991) in different K-complexes preceding arousals. In this work we also showed the deactivation of sigma activity followed by a long-lasting poststimulus inhibition. Because spindles are the expression of the thalamic filter to the passage of stimuli, their transient disappearance could provide a time window for momentarily improved sensorial transmission through the thalamic relay. In spite of the differences in the intensity dimension and in the EEG, autonomic and behavioral components, the variable forms of arousals are supported by a uniform background along a hierarchic continuum (Halász 1998; Sforza et al. 2000b) concerning the degree of activation they produce (Table 3).
ARE THERE ANY INNER SOURCES OF AROUSALS OR ARE THEY ALL COMING FROM OUTSIDE?
In the last 20–30 years of research on the microstructure of sleep EEG and autonomic phenomena a large list of different phasic events, their relationship with each other and with the macrostructure of sleep have been revealed. It has become clear that sleep is scattered by shorter or longer, milder or stronger phasic events. The driving force of these phasic events is obviously a recurring arousal triggered either by unknown origin or by detectable sources outside the sleeper.
Schieber et al. (1971) and later the work of Ehrhart and Muzet (1974) of the same school based especially on the observed equilibrium between the evoked and spontaneous MAs assumed an endogen pacemaker within the RAS, working against sleep promoting influences and becoming more and more active, promoting the awakening process during the last third of night sleep. Regardless of their origin, arousal stimuli exert a general arousal response through the RAS, which is known to be activated by different sensory modalities. McNamara et al. (2002) registered spontaneous arousals in infants between 2 and 10 weeks of age and observed frequent periodic arousals as a sequence involving an augmented breath followed by startle and then cortical arousal, but subcortical arousals were more common than cortical ones. They concluded that there is an endogenous rhythm of spontaneous activity in infants involving excitatory processes from the brainstem, which may or may not be closely followed by cortical excitation.
A well-known example for internal drives of brainstem origin is the ponto-geniculo-occipital (PGO) spike activity detectable in animals before and during REM sleep. PGO waves spontaneously occur in the pons, lateral geniculate body, and occipital cortex during REM sleep, and PGO-like waves may be elicited during sleep and waking by sudden onset stimuli. During REM sleep PGO activity correlates with eye movement bursts. The auditory stimuli eliciting PGO waves during wakefulness produce signs of increased EEG and behavioral arousal, consisting of cortical desynchronization and orienting movements (Kaufmann and Morrison 1981).
It is not definitely clear whether PGO waves exist in humans, but parieto-occipital potentials similar to PGO waves were obtained by averaging EEG segments before and after eye movements during REM sleep (McCarley et al. 1983) and recent functional neuroimaging data indicate a REM-specific positive correlation between rapid eye movements and right geniculate body as well as occipital activity (Peigneux et al. 2001).
The amplitudes of elicited PGO waves in wakefulness were greatest when orienting responses were observed, and the amplitudes of elicited PGO accompanying orienting responses were not significantly different from elicited PGO amplitudes in REM. Likewise, the amplitudes of elicited PGO during REM were not significantly different from those of the highest amplitude spontaneous PGO waves. These findings support the hypothesis that the presence of high-amplitude PGO waves in REM indicates that the brain is in a state of more-or-less continual orienting (Sanford et al. 1993). Both waking and REM sleep PGO waves are related to the pedunculopontine tegmental nucleus that is involved in acoustic and somatosensory stimuli processing (Reese et al. 1995). These data suggest that during REM sleep there is an internal generation of orienting responses similar to those occurring during arousing stimulation in wakeful state (Johnson and Lubin 1967). Orienting responses are also associated with physiologic arousals of NREM sleep, i.e. K-complexes (Campbell et al. 1992) and can occur either spontaneously or be evoked by external stimuli.
This PGO activity could be an internal source of brain activation during REM sleep. This internal arousing system seems to be an inherent part of REM sleep, because auditory stimulation during REM sleep not only increases PGO activity, but also enhances REM sleep duration in cats (Ball et al. 1989; Drucker-Colin et al. 1983) and humans (Mouze- Amady et al. 1986). The triggering neurons of the pontine PGO wave generator are located within the caudolateral peribrachial and the locus subcoeruleus areas, and the transferring neurons of the pontine PGO generator within the cholinergic neurons of the laterodorsal tegmentum and the pedunculopontine tegmentum. Thus PGO activity could be an internal source of brain activation during REM sleep and perhaps even beyond it.
So far, the inner source of arousals periodically appearing during sleep were assumed to be, in the above-mentioned works, in the brainstem, or at least as a result of the interactions between brainstem systems. However, there is another possibility, namely the cyclic variations in cortical excitability providing periodically increased reactivity to any incoming signal.
THE CYCLIC NATURE OF AROUSAL
The sleep process is the outcome of several mechanisms and cyclic phenomena. The rising homeostatic pressure as a function of diurnal waking is compensated by slow wave activity during sleep which undergoes an exponential decline according to the cyclic process S and interacts with the oscillations of the circadian process C (Borbély 1982; Borbely and Achermann 2000; Ferrillo et al. 1991). The ultradian alternation between NREM and REM sleep is another cyclic process that regulates the level of arousability and the degree of EEG synchronization (McCarley and Hobson 1975). Because spontaneous arousals are intimately related to the sleep structure, they are involved in all the mentioned cyclic processes and express an intrinsic rhythm as well. It has been established that approximately 90% of MA are separated by an interval <60 s. In particular, over 70% are separated by an interval between 20 and 40 s (Terzano and Parrino 1991). This means that MA are not randomly distributed but are organized in repetitive sequences based on an approximately 0.033 Hz periodicity (Achermann and Borbély 1997). The CAP is the time domain in which arousal events are grouped in conditions of reduced vigilance. First observed in comatose patients as a cyclic simultaneous variation of EEG patterns and monitored physiologic functions (Fischgold and Mathis 1959; Terzano et al. 1982), CAP was later recognized as a physiolog
ic component of normal NREM sleep (Bruni et al. 2002; Lofaso et al. 1998; Parrino et al. 1998; Terzano and Parrino 2000).
In humans, the existence of a spontaneous ultra-slow rhythm in the CAP range can be identified both in quiet wakefulness and in the sleep condition (Fig. 1). EEG spectral parameters analyzed during a resting period in healthy subjects showed that both theta and alpha band powers oscillate at an average frequency of 0.024 and 0.057 Hz (Novak et al. 1992). A periodicity peaking at approximately 32 s in the domain of slow waves (<4.5 Hz) has been described during NREM sleep in humans (Achermann and Borbély 1997). Another human study reported an ultra-slow rhythm of 0.05–0.025 Hz, which was superimposed on the regular 0.6 Hz EEG rhythms during deep NREM sleep (McKeown et al. 1998). It seems that 20– 40 s periodic changes modulate the background EEG activity and regulate cortical excitability of human subjects.
Another line of evidence is given by animal studies, which report multisecond oscillations corresponding to the CAP range in different areas and conditions. Alternating amplitude segments with a <1 Hz periodicity have been described during NREM sleep in rats (Depootere et al. 1991), and periodic oscillations in EEG and behavioral activity with a cycle length of 15–30 s have been reported in chair-restrained squirrel monkeys. These oscillations consist of alternating episodes of vigilance and inattentiveness, the former characterized by visual scanning and motor movement, the latter by behavioral quiescence (Ehlers and Foote 1984). Oscillations with periods in the 2–60 s range are present in the baseline activity of a majority of basal ganglia neurons recorded in awake immobilized rats (Ruskin et al. 1999). In urethane-anesthetized rats many lateral geniculate neurons display a very slow oscillatory behavior in the range of 0.025–0.01 Hz (Albrecht et al. 1998). A mathematical model of hippocampal function predicted an ultra-slow oscillation in the CA area (Klemm and Naugle 1980), and in fact a 0.025 Hz oscillation in the hippocampus has been recorded from the CA1 and subicular regions in rats of the Wistar and Sprague–Dawley strains, anesthetized with urethane (Penttonen et al. 1999). The very slow oscillatory activity (0.025–0.01 Hz), observed during urethane anesthesia in the lateral geniculate nucleus can be blocked by continuously illuminating the eyes. Light-induced suppression of very slow oscillation could be re-induced by NMDA-antagonists, by non-NMDA antagonists as well as by GABA agonists (Albrecht et al. 1998). In other words, powerful stimulation suppresses and pharmacologic inhibition re-induces the very slow oscillatory activity. These results suggest that oscillatory activity in the CAP range could be a general property of central nervous system function during periods of reduced arousal, and that this oscillatory activity, EEG expression of unstable vigilance, can be manipulated by sensorial inputs.
During sleep, an acoustic stimulus delivered during stable non-CAP (Fig. 1) can evoke a prolonged CAP sequence (Terzano and Parrino 1991). Accordingly, the amount of CAP increases when sleep is achieved under conditions of noise stimulation (Terzano et al. 1990, 1993). CAP rate (the ratio of CAP time to NREM sleep time) also surges in situations of sleep disruption, such as psychophysiologic (Terzano and Parrino 1992; Terzano et al. 1997a) and organic (Szűcs et al. 2000) insomnia, while it is lowered by sleep-promoting conditions and sedative pharmacologic treatment (Parrino and Terzano 1996; Parrino et al. 1997). Accordingly, CAP rate is decreased by night-time recovery sleep after total sleep deprivation (De Gennaro et al. 2002; Parrino et al. 1993) and by hypnotic medication (Terzano et al. in press). During NREM sleep, the phase A of CAP triggers and modulates the distribution of epileptic events (Halász et al. 2002; Parrino et al. 2000a) and myoclonic jerks (El-Ad and Chervin 2000; Haba-Rubio et al. 2002; Mahowald2002; Parrino et al. 1996). Similar observations were found for the ultra-slow oscillation in the hippocampal CA1 region, which consists of an alternation of network excitability, triggering epileptic-like after discharges during phases of enhanced network excitability in susceptible rat strains (Penttonen et al. 1999). In contrast, phase B of CAP is closely related to the repetitive respiratory events of sleep-disordered breathing (Parrino et al. 2000a; Terzano et al. 1996; Thomas 2002), and only the powerful autonomic activation during the following CAP-A phase can restore postapnea breathing (Parrino et al. 2000b; Terzano et al. 1996). These observations suggest that CAP-A is the activation phase, which alternates with a reduced neural excitability characterizing CAP-B phases.
All these results indicate that both spontaneous and elicited phasic arousals, especially during NREM sleep, have a cyclic nature following the multisecond oscillation. As a translation of fluctuating arousal, CAP offers a favorable background for sleep disorder manifestations (e.g. epileptic abnormalities, PLM, nocturnal apneas, NREM parasomnias, insomnia) which are related to a condition of unstable sleep (Terzano and Parrino 1993a) during which CAP cycles play a promoting (phase A) or a dysfacilitating (phase B) gating action on the single EEG, behavioral and autonomic events.
Besides CAP, the other major EEG activity in the frequency range below 1 Hz, characterizing states of reduced tonic arousal, is the so-called slow oscillation (Steriade et al. 1993). This 0.5–0.9 Hz EEG rhythm was outlined by deep EEG recordings performed during anesthesia and NREM sleep of cats and rats (Steriade 2000b) as well as by surface EEG and magnetoencephalography during NREM sleep of human subjects (Achermann and Borbély 1997; Amzica and Steriade 1997; Simon et al. 2000). Slow oscillation is generated in cortical neurons, and consists of phases of depolarization, characterized by intensive neural firing, followed by long-lasting hyperpolarization (Steriade 2000a). Hence the two phases of slow oscillation are characterized by opposite neural phenomena: cortical excitation made up of synaptic potentials and cortical inhibition mainly due to network dysfacilitation. The excitatory component of slow oscillation is effective in grouping the K-complexes and delta waves, which do not occur in isolation but are grouped into complex wave sequences. The coalescence of slow rhythms is especially visible during NREM sleep (Steriade and Amzica 1998). However, it is known that periodic K-complexes and delta bursts, which coalesce within CAP, are the basic components of phase A1 subtypes. The other phase A subtypes (A2 and A3), instead, encompass not only slow rhythms but also fast activities in different proportions. In experimental studies, fast rhythms at 30–40 Hz, mainly described in the wake state and in REM sleep, are present during NREM sleep as well (Steriade 1995). The prerequisite of fast rhythms and cortical activation is provided once again by the slow (<1 Hz) cortical oscillation, which permits fast oscillation to occur periodically, just timing with the occurrence of K-complexes (Amzica and Steriade 1997). This can explain why K-complexes can be associated often not only with slow activities, i.e. K-delta but also with rapid activities, e.g. K-alpha and polyphasic bursts (Halász 1993). According to these data there is a periodic window in NREM sleep, which allows, like an alternatively opening and closing communication pore, the possibility for the brain to be activated by sensory input. When the incoming sensory bombardment coincides with the window opening the conditions for activation are present. This explains why periodic arousals are so closely associated with K-complexes, appearing in the garment of sleep rhythms.
Another biologic hypothesis regarding arousal periodicity during NREM sleep is to allow sleeping animals’ opportunities to monitor their environment and thus avoid predation. The periodic neuronal excitation serves as a sentinel
function in a state of disconnection from the external world and sets the cerebral functions in a readiness state for a possible adaptive behavior. CAP can be enhanced by any internal or external factor of perturbation. When a disturbing factor is administered to a sleeping brain, a poststimulus CAP sequence can persist for some minutes. However, CAP appears even in the absence of any environmental disturbance acting as a structural component of physiologic sleep intervening in close temporal relation with stage shifts and body movements (Terzano et al. 1988).
PATHOLOGIC AROUSALS
Spontaneous arousals are natural guests of the sleeping brain (Boselli et al. 1998) and appear regularly embedded within the CAP process (Parrino et al. 2001; Terzano et al. 2002). However, arousal phenomena are also known to occur in response to sleep-disturbing factors. Increased amounts of arousals are a regular finding of obstructive sleep apnea syndrome (OSAS), but typical manifestations of secondary cortical events are also the respiratory effort-related arousals (RERA) known as obstructed events that do not meet the criteria for apnea or hypopnea but that nevertheless cause an arousal. More specifically, RERA are defined as the absence of apnea/hypopnea but with progressive negative Pes (esophageal pressure) lasting ‡10 s culminating in an arousal. RERA are increased both in OSAS and in the upper airway resistance syndrome (UARS) as a reaction of the sleeping brain to a repetitive breathing disturbance. RERA are secondary to subtle obstructions of the upper airway during sleep and can appear in the absence of apneas and hypopneas, causing excessive daytime sleepiness. The common abundance of RERA in sleep-disordered breathing (SDB) has supported the idea that arousals are a sign of disturbed sleep and that arousal responses reflect abnormal breathing (Douglas 2000). The association between esophageal pressure alterations and arousals even in the absence of apneas or hypopneas justifies this belief and has been one of the main reasons for considering arousals as an epiphenomenon of SDB. However, arousals can be elicited by non-respiratory disturbance. Besides respiratory-driven, the so-called spontaneous arousals could in effect be supported by some organic trigger such as intestinal passage, excessive bladder loading or organ dysfunction. Accordingly, the occurrence and distribution of these events should follow the randomness of internal phenomena across the night. However, if we verify the position of non- pathologic arousals during sleep we can ascertain that they are not randomly distributed as they tend to vanish in the first part of the DS (Evans 1993) and appear mainly concentrated in the AS of the sleep cycle (Halász et al. 1979; Terzano et al. 2000). In particular, arousals commonly occur before and during REM sleep but are rare during slow wave sleep (Bonnet and Arand 1997b; Ehrhart and Muzet 1974). These findings indicate that the occurrence of a certain amount of arousals is related to the intrinsic organization of sleep regardless of any superimposed source of disturbance. Accordingly, discrimination between spontaneous arousals and induced arousals can be more reliably ascertained if we are able to identify which and where are the arousals that belong to the physiologic structure of sleep. This implies that when sleep is not severely disrupted, pathologic arousals tend to appear in those portions of sleep in which they have higher probabilities to occur spontaneously. In SDB, a certain amount of respiratory- induced arousals may simply replace the spontaneous ones as expected from their natural distribution across the night. It has been ascertained that a CAP sequence preceding the onset of REM sleep is a structural marker of sleep organization (Terzano et al. 1988). This means that this transitional portion of sleep coexists with an underlying oscillation of vegetative functions. The occurrence of unstable sleep in this particular position within the sleep cycle can be an important factor for monitoring respiratory oscillations and titrating ventilatory support (Thomas 2002). Moreover, Poyares et al. (2002) hypothesize an inability of the CNS to manifest spontaneous arousals when they are already occurring quite often due to a specific disturbance. Acoustic stimulation during sleep increases the amount of noise- induced arousals and reduces the amount of spontaneous arousals (Halász et al. 1979). These findings indicate a mutual influence between physiologic and pathologic arousals.
Secondary arousals are not linked only to breathing abnormalities but can also occur in association with motor phenomena. Sleep bruxism is a typical form of oromotor activity associated with sleep arousals (Kato et al. 2003; Macaluso et al. 1998). Muscle jerks occurring in close temporal relations with arousals are typical manifestations of periodic limb movement. Painful syndromes are also commonly associated with increased arousals (Lavigne et al. 2000; Parrino et al. 2003). However, increased arousals can occur in clinical conditions lacking any detectable internal or external factor of perturbation. Primary insomnia, a sleep disorder without any evidence of mental, substance-induced or medical disturbance, shows increased amounts of arousals and CAP compared with sound sleepers (Terzano et al. in press).
WHEN TOO MANY AROUSALS DETERMINE NON-RESTORATIVE SLEEP
There is a great body of evidence that sleep fragmentation – punctuation of sleep with frequent, brief arousals – diminishes its recuperative value (Bonnet 1985, 1986; Stepanski et al. 1987). This statement is valid even when these arousals do not alter the standard 30-s epoch sleep stage scoring. In other words too many arousals can impair sleep continuity even when sleep efficiency is preserved. For a long time, in disorders of sleep maintenance or those conditions in which sleep was felt to be not enough satisfactory and recuperative, nothing wrong was possible to detect when sleep was analyzed through the traditional scoring system (Rechtschaffen and Kales 1968). Only after the introduction of microstructural analysis of sleep it became possible to find parameters changing parallel with the subjective complaints of insomniac patients. Sleep of insomniacs contains more sleep instability as measured by CAP parameters (Paiva et al. 1993; Terzano and Parrino 1992). In primary insomnia, all phase A subtypes are increased including subtypes A3 (which coincide with arousals). However, when these patients are treated with hypnotic agents and report a significant improvement of sleep quality there is a parallel reduction of CAP rate associated with an important reduction of subtypes A1 and A2 (Terzano et al. in press).
In normal sleepers, noise-induced delta bursts, corresponding to subtypes A1 and A2, reduce the restorative properties of sleep and determine excessive daytime sleepiness even when there is no evidence of sleep fragmentation (Martin et al. 1997b; Terzano and Parrino 1991; Terzano et al. 1990). Accordingly, we can assume that a condition of disturbed sleep can be associated with a paradoxical increase of delta activity, which is considered as an expression of restorative sleep (Borbely and Achermann 2000).
In general, arousal responses differ in the specific sleep disorders. The association between sleep-related respiratory events and EEG arousals is more frequently reported in OSAS than in UARS. This is likely because OSAS subjects present increase in effort accompanied by apneas and hypopneas, and sometimes by short and limited oxygen saturation drops, requiring a more intense stimulus to arouse. Correlation between the number of arousals and daytime sleepiness in OSAS patients has been reported (Sforza and Lugaresi 1995; Zucconi et al. 1995a), but the activating role of phasic delta activities during sleep (with diurnal consequences) should be emphasized. However, Black et al. (2000) and Poyares et al. (2002) have found that airway opening may occur in UARS subjects with a predominant increase in delta p
ower. In other words, reopening of the airway at wakefulness and disappearance of abnormal UARS are not necessarily associated with an arousal. Reopening of the airway with an EEG pattern of delta has been also observed in OSAS patients (Berry et al. 1998). Involvement of either slow or fast EEG responses depends on the regulation of upper airway pathway. Respiratory patterns that need correction activate the CNS. This activation varies, depending on the sensory recruitment and the adequacy of the response. A respiratory challenge can be resolved by CNS activation without involving a cortical arousal. The latter is triggered only when thalamo-cortical structures fail to modulate breathing or when ascending reticular volleys are required to restore respiration (Hirshkowitz 2002). Depending on the amount of recruitment and numbers of neural structures involved, the CNS activation will be variable. The autonomic nervous system is enhanced when an arousal occurs, which explains the greater increase in heart rate with EEG arousal than without EEG arousal. Anyway, the problem is quantitative not qualitative in the sense that delta bursts can also determine heart rate acceleration and autonomic activation (Sforza et al. 2000a). Generally, the slow and the fast components of EEG activation have different latencies, with the delta portion preceding the rapid activities (De Carli et al. in press). This probably determines a graduated impact on the autonomic system. The slow waves determine a softer vegetative reaction, which in certain pathologic conditions may be strong enough to overcome a disturbing factor, e.g. an obstructive event. Otherwise, the slow rhythms are immediately replaced by faster EEG activities, which guarantee a more powerful activation of autonomic functions. Probably the effects on daytime function is not linked to a single phase A subtype but to the reciprocal amount and distribution of the single subtypes. In OSAS patients treated effectively with nasal CPAP, ventilatory- induced reduction of CAP rate, which correlates significantly with daytime sleepiness, was associated with a robust curtailment of subtypes A3 and an expansion of the A1 percentage (Parrino et al. 2000b). Further studies will clarify the relationship between sleep microstructure and diurnal wellness.
AROUSAL DISORDERS
Arousal disorders were first delineated by Broughton (1968). He assumed that abnormality of the arousal process prevents the normal arousal response from NREM that otherwise would lead to full alertness and results in a pathologic arousal, a dissociated state, amalgamating features of sleep and wakefulness, in certain disorders. Disorders grouping together by this assumed pathomechanism are known as sleepwalking (SW) and sleep terror (ST) and a milder form of the continuum of these disorders, named confusional arousal (CA). All three of them has a beginning in childhood before puberty and prevails in young adulthood. The common symptoms of the dissociated state observed during these pathologic arousals are: (a) mental confusion and disorientation, (b) automatic behavior, (c) relative non-reactivity to external stimuli, (d) poor response to efforts to provoke behavioral wakefulness, (e) retrograde amnesia for many intercurrent events, (f) only fragmentary or no recall of dream mentation (Thorpy 1990).
Macrostructure of sleep is preserved, but studying the microstructure, several pathologic features were observed in different works. NREM sleep in adults has shown increased number of MA preceded and accompanied by EEG synchronization (Halász et al. 1985a; Zucconi et al. 1995b). Presence of hypersynchronous delta (HSD) wave activity and increased sleep instability and arousal oscillations, reflected in an increased CAP rate (Zucconi et al. 1995a) were also reported. An analysis of the pathologic behavioral events (SW and/or ST) of 19 polysomnographic records in 12 adults showing 252 arousals from NREM sleep, compared with a control group, showed delta wave clusters in 15.6% of pre-event periods and in 37.9% immediately preceding the slow-wave sleep MA (Schenck et al. 1998). The arousal period was characterized by three kinds of EEG symptoms: (a) diffuse rhythmic delta activity around4 Hz, most prominent in bilateral anterior regions, typically 20 s duration; (b) diffuse and irregular, moderate to high voltage delta and theta activity with anterior dominance intermixed with, or superimposed by, alpha and beta frequencies; (c) prominent alpha and beta activity, at times intermixed with moderate voltage thetas. Nearly half of the patients showed one of the first two types of EEG pattern. All the three types of EEG patterns were associated with variable degrees of heart rate acceleration with a shortening of R-R interval. It is clear from the report that a slow wave type of MA characterizes pathologic arousal itself in half of the patients. We do not know how much we should regard this type of arousal from NREM sleep as pathologic in this age group, compared, for example, with an abrupt arousal from NREM sleep, due to a sudden pain. It is an everyday experience for electroencephalographists dealing with sleep records that this kind of delta synchronization occurs frequently in slow wave arousals of children or adolescents, but it is not known how much the maintenance of this type of arousal to adulthood should be interpreted as pathologic.
Guilleminault et al. (2001) explored the spectral features of EEG in SW patients across sleep cycles and immediately preceding a CA. They confirmed an increased MA rate during the first cycles of sleep and found an important increase in relative power of low delta (0.75–2 Hz) just prior to the SW episodes. They assumed that it is the ‘cortical reaction to brain activation’ which would serve to avoid the interruption of sleep and in the same time may be responsible for the confused state. Gaudreau et al. (2000) reported similar findings suggesting that arousal disorders reflect an exaggeration of the antiarousal defense mechanisms mainly restricted to the DS of first sleep cycles. These findings again show that in NREM sleep delta arousal is one of the possible forms of activation, and it is questionable how much these slow wave arousals can be held as pathologic arousals or are only the childhood form of arousal preserved in adulthood, as proposed above.
AROUSALS GATING PATHOLOGIC EVENTS
Among pathologic arousals gating effect has the more extended literature. Several pathologic sleep events were found to be associated with different forms of MA. The most explored sleep perturbation in association with different sleep pathologies is the CAP pattern. Within the CAP it is mostly the A-phase that is connected with these abrupt manifestations of pathologic sleep events. Therefore CAP A-phase was interpreted as a kind of ‘gate’ through which pathologic events occur more easily. The gating effect has been demonstrated in the last years among several sleep disturbances such as PLM (El-Ad and Chervin 2000; Haba-Rubio et al. 2002; Parrino et al. 1996), sleep bruxism (Kato et al. 2003), OSAS (Terzano and Parrino; Thomas 2002) and epilepsy (Eisensehr et al. 2001; Halász et al. 1985a, 2002; Terzano et al. 1989, 1991b, 1997b).
CONCLUSIVE REMARKS ON THE FUNCTIONS OF AROUSAL DURING SLEEP
The data available on arousal activity during NREM sleep clearly indicate that arousal is really woven into the texture of sleep. What are the functions of the ongoing arousal activity during NREM sleep, the essence of which is conventionally held just the opposite to arousal? In general, arousals and arousability ensure the reversibility of sleep, without which it would be identical to coma. Arousals provide a connection of the sleeper with the surrounding world maintaining the selection of relevant incoming information and adapting the organism to the dangers and demands of the outer world. In this dynamic perspective, the ongoing phasic events carry on the one hand arousal influences and on the other elements of information processing. Therefore, arousal and inform
ation processing are the two sides of the same coin in sleep. The latter statement is supported by the increasing investigation of different components of K-complexes and their relationship with the presence/absence and different features of cognitive workup during NREM sleep (Atienza et al. 2001; Bastuji and Gracia-Larrea 1999; Niiyama et al. 1995; Perrin et al. 1999, 2000; Sallinen et al. 1994).
The other function of arousals is tailoring the more or less stereotyped endogenously determined sleep process driven by chemical influences according to the internal and external demands. This is why the sleep process is variable from night to night, lending flexibility to the process. Different forms of arousals provide the phasic regulation prevailing on top of the slower waves of pre-programmed chemical codes, shaping in a certain limited way the sleep process. This regulation is able to modify mainly the AS of the sleep cycles and prevails in the last third of the night sleep.
Speculations on how sleep and wakefulness are regulated consider homeostatic and circadian factors as essential to explain the timing of alternations of awake state and sleep (Borbély 1982), while reciprocal interactions of brainstem neuronal systems are thought to be involved in the alternations of NREM and REM sleep (Hobson and McCarley 1977; McCarley and Massaquoi 1992). In this perspective, arousals shape the individual course of night sleep as a variation of the sleep program (McCarley and Massaquoi 1992; Massaquoi and McCarley 1992). Based on the data gathered from the study of MA it seems plausible instead those arousals have a more essential role in the reciprocal interactions between NREM sleep and wake and between NREM sleep and REM sleep.
We can envisage control of sleep/wakefulness as a tonic regulation under endogenously driven reciprocal antagonistic chemical influences like the promoted ‘sleep switch’ (flip-flop) model based on the hypothalamic control of sleep and wakefulness (Saper et al. 2001). However, this interpretation cannot explain the intermediate states and flexibility of the system. This is assured by the parallel ‘phasic’ regulation provided by arousals in sleep, proposed to be incorporated in the current models of sleep regulation. Fig. 6 shows schematically the parallel working mode of ‘tonic’ modulation (mainly intracerebral, slow, and chemical) and ‘phasic’ changes (mainly extracerebral, faster, and neuronal-synaptic). The sleep promoting system (S) and arousal system (A) exert mutual reciprocal antagonistic influences on each other. The detailed description of this relationship and the role of the players (both chemical and neural) of the two interacting subsystems are brilliantly illustrated in Saper et al. (2001). In the DS of sleep cycles the S system is dominating. When the S system inhibits the A system to a certain extent (during DS) sensorial input from the inner and outer surrounding (interrupted thick arrows) result in rare and mild arousals. In contrast, during the AS the tonic inhibition of the A system decreases and this facilitates a greater frequency of arousals. The continuous arrows represent the endogenous chemical and the interrupted arrows the sensorial inflow. The thickness of the arrows reflects the proportion of the relations. This model is able to explain why sleep gets deeper during the DS even in the presence of sensory stimulation (Hirshkowitz 2002), and how arousals can promote, during the AS, the avalanche of the awakening process. Here the dynamic changes across the sleep cycle are fueled not only by chemical influences but also by the parallel sensorial input which has state-specific different functions during the two slopes of the cycle, being sleep promoting during DS and supporting the arousal process during AS. The sleep-promoting effect of sensory stimuli during the process of falling asleep has been reported by several authors (Bohlin 1971; Oswald 1960; Webb and Agnew 1981), starting from Pavlov (1928).
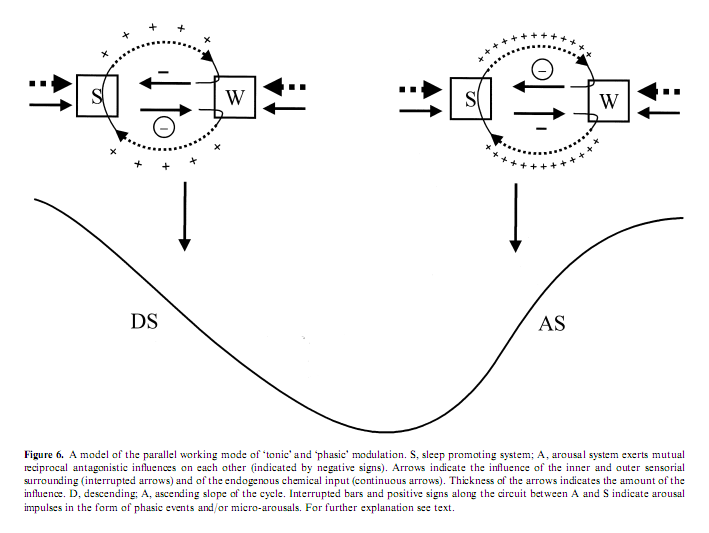
Every biologic system tries to assure autonomy to achieve independence from the surrounding, and at the same time relies on the interrelationship between the organism and the surrounding world, which is essential for adaptation and survival of the system. Therefore an organism should avoid external stimuli and try to regain the original prestimulus state, but paradoxically, it will use the stimulus for building up its autonomic state. The reciprocal interplay of the sleep/wakefulness system is a suitable example of how external stimuli are used in a process modeling the internal structure and serving the separation of the organism from the outer world (Atlan 1979).
Anyway, the sleeping brain can offer manifold types of MA to internal or external inputs. Besides the classical low-voltage fast-rhythm EEG arousals, high-amplitude EEG bursts, be they like delta-like or K-complexes, reflect a possible arousal process. Different MAs are associated with increasing magnitude of vegetative activation ranging hierarchically from the weaker slow EEG types (coupled with mild autonomic activation) to the stronger rapid EEG types (coupled with a vigorous autonomic activation). In physiologic conditions, slow and fast MAs are not randomly scattered but appear structurally distributed within sleep where they are also endowed with a cyclic nature expressed by the periodic dimension of CAP. It is known that arousal responses differ in various sleep disorders. Understanding the role of arousals and CAP and the relationship between physiologic and pathologic MA can shed light on the adaptive properties of the sleeping brain and provide insight into the pathomechanisms of sleep disturbances.
REFERENCES
Achermann, P. and Borbély, A. A. Low-frequency (<1 Hz) oscillations in the human sleep electroencephalogram. Neuroscience, 1997, 81: 213–222.
Ackner, B. and Pampiglione, G. Some relationships between peripheral vasomotor and EEG changes. J. Neurol. Neurosurg. Psychiat., 1957, 20: 58–64.
Albrecht, D., Royl, G. and Kaneoke, Y. Very slow oscillatory activities in lateral geniculate neurons of freely moving and anesthetized rats. Neurosci. Res., 1998, 32: 209–220.
Amzica, F. and Steriade, M. The K-complex: its slow (<1-Hz) rhythmicity and relation to delta waves. Neurology, 1997, 49: 952– 959.
Atienza, M., Cantero, J. L. and Escera, C. Auditory information processing during human sleep as revealed by event-related brain potentials. Clin. Neurophysiol., 2001, 112: 2031–2045.
Atlan, H. Entre le cristal et la fumée. Essai sur l’organisation du vivant. Seuil. Ed., Paris, 1979.
Atlas Task Force. EEG arousals: scoring rules and examples. A preliminary report from the sleep disorders Atlas Task Force of the American Sleep Disorders Association. Sleep, 1992, 15: 174–184.
Ball, W. A., Morrison, A. R. and Ross, R. J. The effect of tones on PGO waves in slow wave sleep and paradoxical sleep. Expt. Neurol., 1989, 104: 251–256.
Bastien, C. and Campbell, K. The evoked K-complex: all-or-none phenomenon? Sleep, 1992, 15: 236–245.
Bastuji, H. and Garcia-Larrea, L. Evoked potentials as a tool for the investigation of human sleep. Sleep Med. Rev., 1999, 3: 22–45.
Bastuji, H., Garcia-Larrea, L., Franc, C. and Maugiere, F. Brain processing of stimulus deviance during slow-wave and paradoxical sleep: a study of human auditory evoked responses using the oddball paradigm. J. Clin. Neurophysiol., 1995, 12: 155–167.
Berger, H. Ueber das Elektroenkephalogramm des Menschen. J. Psychol. Neurol., 1930, 40: 160–179.
Berry, R. B., Asyali, M. A., Mcnellis, M. I. and Khoo, M. C. Within night variation in respiratory effort preceding apnea termination and EEG delta power in sleep apnea. J. Appl. Physiol., 1998, 85: 1434– 1441.
Black, J. E., Guilleminault, C., Colrain, I. M. and Carrillo, O. Upper airway resistance syndrome. Central electroencephalog
raphic power and changes in breathing effort. Am. J. Respir. Crit. Care Med., 2000, 162: 406–411.
Bohlin, G. Monotonous stimulation, sleep onset and habituation of the orienting reaction. EEG Clin. Neurophysiol., 1971, 31: 593–601. Bonnet, M. H. Cumulative effects of sleep disruption on performance, sleep and mood. Sleep, 1985, 8: 11–19.
Bonnet, M. H. Cumulative effects of sleep restriction on daytime sleepiness. Physiol. Behav., 1986, 37: 915–919.
Bonnet, M. H. and Arand, D. L. Heart rate variability: sleep stage, time of night, and arousal influences. Elec. Clin. Neurophysiol., 1997a, 102: 390–396.
Bonnet, M. H. and Arand, D. L. The distribution of arousals in normal sleep. APSS 11th Annual Meeting. Abstr. Book, 1997b: 557.
Borbély, A. A. A two-process model of sleep regulation. Hum. Neurobiol., 1982, 17: 449–455.
Borbely, A. A. and Achermann, P. Sleep homeostasis and models of sleep regulation. In: M. H. Kryger, T. Roth and W. C. Dement (Eds) Principles and Practice of Sleep Medicine, 3rd edn. W. B. Saunders Company, Philadelphia, 2000: 377–390.
Boselli, M., Parrino, L., Smerieri, A. and Terzano, M. G. Effect of age on EEG arousals in normal sleep. Sleep, 1998, 21: 351–357.
Braun, A. R., Balkin, T. J., Wesenten, N. J., Carson, R. E., Varga, M., Baldwin, P., Selbie, S., Belenky, G. and Herscovitch, P. Regional cerebral blood flow throughout the sleep–wake cycle. An H2(15)O PET study. Brain 1997, 120: 1173–1197.
Braun, A. R., Balkin, T. J., Wesensten, N. J., Gwadry, F., Carson, R. E., Varga, M., Baldwin, P., Belenky, G. and Herscovitch, P. Dissociated pattern of activity in visual cortices and their projections during human rapid eye movement sleep. Science, 1998, 279: 91–95.
Bremer, F. Cerveau ‘isolé’ et physiologie du sommeil. C. R. Soc. Biol. (Paris), 1935, 118: 1235–1241.
Bremer, F. and Stoupel, N. Facilitation and inhibition synaptic linkages disrupt synchronization of a slow oscillation. J. Neurosci., 1959, 67: 240–275.
Broughton, R. J. Sleep disorders: disorders of arousal? Science, 1968, 159: 1070–1078.
Broughton, R. J., Poiré, R. and Tassinari, A. The electrodermogram (Tarchanoff effect) during sleep. EEG Clin. Neurophysiol., 1965, 18: 691–708.
Bruni, O., Ferri, R., Milano, S., Verrillo, E., Vittori, E., Della Marca, G., Farina, B. and Mennuni, G. Sleep cyclic alternating pattern in normal school-age children. Clin. Neurophysiol., 2002, 113: 1806–1814.
Buchsbaum, M. S., Gillin, J. C., Wu, J., Hazlett, E., Sicotte, N., Dupont, R. M. and Bunney, W. E. Jr. Regional cerebral glucose metabolic rate in human sleep assessed by positron emission tomography. Life Sci., 1989, 45: 1349–1356.
Campbell, K., Bell, I. and Bastien, C. Evoked potential measures of information processing during natural sleep. In: R. J. Broughton and R. D. Ogilvie (Eds) Sleep, Arousal, and Performance. Birkhauser, Boston, MA, 1992: 88–116.
Cantero, J. L. and Atienza, M. Alpha burst activity during human REM sleep: descriptive study and functional hypotheses. Clin. Neurophysiol., 2000, 111: 909–915.
Carley, D. W., Applebaum, R., Basner, R. C., Önal, E. and Lopata, M. Respiratory and arousal responses to acoustic stimulation. Chest, 1997, 112: 1567–1571.
Curzi-Dascalova, L. and Dreyfus-Brisac, C. Distribution of skin potential responses according to states of sleep during the first months of life in human babies. EEG Clin. Neurophysiol., 1976, 41: 399–407.
Curzi-Dascalova, L., Pajot, N. and Dreyfus-Brisac, C. EDG spontaneous activity and sleep stages in premature infants. Polygraphic study. Rev. Neurol. (Paris), 1970, 123: 231–239.
De Gennaro, L., Ferrara, M. and Bertini, M. The spontaneous K- complex during stage 2 sleep: is it the ‘forerunner’ of delta waves? Neurosci. Lett., 2000, 291: 41–43.
De Gennaro, L., Ferrara, M., Spadini, V., Curcio, G., Cristiani, R. and Bertini, M. The cyclic alternating pattern decreases as a consequence of total sleep deprivation and correlates with EEG arousals. Neuropsychobiology, 2002, 45: 95–98.
De Carli, F., Nobili, L., Beelke, M., Watanabe, T., Smerieri, A., Parrino, L., Terzano, M. G. and Ferrillo, F. Quantitative analysis of sleep EEG microstructure in the time-frequency domain. Brain Res. Bull. (in press).
Dempsey, E. W., Morison, R. S. and Morison, B. R. Some afferent diencephalic pathways related to cortical potentials in the cat. Am. J. Physiol., 1941, 131: 718–731.
Depootere, H., Granger, P., Leonardon, J. and Terzano, M. G. Evaluation of cyclic alternating pattern in rats by automatic analysis of sleep amplitude variations. Effect of zolpidem. In: M. G. Terzano, P. Halász and A. C. Declerck (Eds) Phasic Events and Dynamic Organization of Sleep. Raven Press, New York, 1991: 17–33.
Dijk, D. I., Brunner, D. P. and Borbely, A. A. Time course of EEG power density during long sleep in humans. Am. J. Physiol., 1990, 258: 650–651.
Douglas, N. Respiratory physiology: control of ventilation. In: M. H. Kryger, T. Roth and W. C. Dement (Eds) Principles and Practice of Sleep Medicine, 3rd edn. W. B. Saunders Company, Philadelphia, 2000: 221–241.
Drucker-Colin, R., Bernal-Pedraza, J., Fernandez-Cancino, F. and Morrison, A. R. Increasing PGO spike density by auditory stimulation increases the duration and decreases the latency of rapid eye movement (REM) sleep. Brain Res., 1983, 278: 308–312.
Ehlers, C. L. and Foote, S. L. Ultradian periodicities in EEG and behavior in the squirrel monkey (Saimiri sciureus). Am. J. Primatol., 1984, 7: 381–389.
Ehrhart, J. and Muzet, A. Fréquence et durée des phases d’activation transitoire au cours du sommeil normal chez L’homme. Arch. Scr. Physiol., 1974, 28: 213–260.
Eisensehr, I., Parrino, L., Noachtar, S., Smerieri, A. and Terzano M. G. Sleep in Lennox-Gastaut syndrome: the role of the cyclic alternating pattern (CAP) in the gate control of clinical seizures and generalized polyspikes. Epilepsy Res., 2001, 46: 241–250.
El-Ad, B. and Chervin, R. D. The case of a missing PLM. Sleep, 2000, 23: 450–451.
Evans, B. M. Cyclical activity in non-rapid eye movement sleep: a proposed arousal inhibitory mechanism. EEG Clin. Neurophysiol., 1993, 86: 123–131.
Ferini-Strambi, L., Bianchi, A., Zucconi, M., Oldani, A., Castronovo, V. and Smirne, S. The impact of cyclic alternating pattern on heart rate variability during sleep in healthy young adults. Clin. Neurophysiol., 2000, 111: 99–101.
Ferri, R., Parrino, L., Smerieri, A., Terzano, M. G., Elia, M., Musumeci, S. A. and Pettinato, S. Cyclic alternating pattern and spectral analysis of heart rate variability during normal sleep. J. Sleep Res., 2000, 9: 13–18.
Ferri, R., Cosentino, F. I., Elia, M., Musumeci, S. A., Marining, R., and Bergonzi, P. Relationship between delta, sigma, beta and gamma EEG bands at REM sleep onset and REM sleep end. Clin. Neurophysiol., 2001, 112: 2046–2052.
Ferrillo, F., De Carli, F., Manfredi, C., Zat, C. and Rosadini, G. Analysis of the spectrum of sleep EEG structure. In: S. Smirne, M. Franceschi and L. Ferini-Strambi (Eds) Sleep and Ageing. Masson, Milano, 1991: 41–45.
Ferrillo, F., Gabarra, M., Nobili, L., Parrino, L., Schiavi, G., Stubinski, B. and Terzano, M. G. Comparison between visual scoring of cyclic alternating pattern (CAP) and computerized assessment of slow EEG oscillations in the transition from light to deep non-REM sleep. J. Clin. Neurophysiol., 1997, 14: 210–216.
Fischgold, H. and Mathis, P. Obnubilations, comas et stupeurs Etudes Electroenceph. Masson et Cie, Paris, 1959.
Freixa, I. B. E., Chevalier, B., Grubar, J. C., Lambert, J. C., Lancry, A., Leconte, P., Meriaux, H. and Spreux F. Spontaneous electrodermal activity during sleep in man: an intranight study. Sleep, 1983, 6: 77–81.
Fruhstorfer, H., Partanen, J. and Lumio, J. Vertex sharp waves and heart action during the onset of sleep. E
EG Clin. Neurophysiol., 1971, 31: 614–617.
Garcia-Larrea, L., Perrin, F. and Bastuji, H. Memory encoding, stimulus awareness and event-related potentials. Lessons from a forced awakening test. Clin. Neurophysiol., 2002, 113: S34.
Gaudreau, H. S., Zadra, A. and Montplaisir, J. Dynamics of slow- wave activity during the NREM sleep of sleepwalkers and control subjects. Sleep, 2000, 23: 755–760.
Guilleminault, C. and Stoohs, R. Arousal, increased respiratory efforts, blood pressure and obstructive sleep apnoea. J. Sleep Res., 1995, 4: 117–124.
Guilleminault, C., Poyares, F. and Abat, L. P. Sleep and wakefulness in somnambulism: a spectral analysis study. J. Psychosom. Res., 2001, 51: 411–416.
Haba-Rubio, J., Staner, L. and Macher, J. P. Periodic arousals or periodic limb movements during sleep? SleepMed., 2002, 3: 517–520.
Halász, P. The role of the nonspecific phasic activation in the sleep regulation and in the mechanism of generalised epilepsy with spike- wave pattern. Academic doctoral thesis, Semmelweis University, Budapest, 1982.
Halász, P. Arousals without awakening-dynamic aspect of sleep. Physiol. Behav., 1993, 54: 795–802.
Halász, P. Hierarchy of micro-arousals and the microstructure of sleep. Neurophysiol. Clin., 1998, 28: 461–475.
Halász, P. and Ujszászi, J. Spectral features of evoked micro-arousals. In: M. G. Terzano (Ed.) Phasic Events and Dynamic Organization of Sleep. Raven Press, New York, 1991: 85–100.
Halász, P., Kundra, O., Rajna, P., Pal, I. and Vargha, M. Micro- arousals during nocturnal sleep. Acta Physiol. Acad. Sci. Hung., 1979, 54: 1–12.
Halász, P., Rajna, P., Pál, I., Kundra, O., Vargha, M. and Aune, Gy. Viharos electrodemographiás tevékenység mély alvásban – kísérlet a jelenség magyarázatára. Magyar Pszichológiai Szemle, 1980, 37: 127–139.
Halász, P., Pál, I. and Rajna, P. K-complex formation of the EEG in sleep: a survey and new examinations. Acta Physiol. Acad. Sci. Hung., 1985a, 65: 3–35.
Halász, P., Ujszászi, J. and Gádoros, J. Are microarousals preceded by electroencephalographic slow wave synchronization precursor of confusional awakening? Sleep, 1985b, 8: 231–238.
Halász, P., Terzano, M. G. and Parrino, L. Spike-wave discharge and the microstructure of sleep-wake continuum in idiopathic generalised epilepsy. Neurophysiol. Clin., 2002, 32: 38–53.
Hirshkowitz, M. Arousals and anti-arousals. Sleep Med., 2002, 3: 203– 204.
Hobson, A. J. Toward a cellular neurophysiology of the reticular function: conceptual and methodological milestones. In: A. J. Hobson and A. B. Brazier (Eds) The Reticular Formation Revisited. Raven Press, New York, 1978: 7–29.
Hobson, J. A. and McCarley, R. W. The brain as a dream state generator: an activation-synthesis hypothesis of the dream process. Am. J. Psych., 1977, 134: 1335–1348.
Hobson, J. A., McCarley, R. W. and Wyzinski, P. W. Sleep cycle oscillation: reciprocal discharge by two brainstem neuronal groups. Science, 1975, 189: 55–58.
Horner, R. L., Stanford, L. D., Pack, A. I. and Morrison, A. R. Activation of a distinct arousal state immediately after spontaneous awakening from sleep. Brain Res., 1997, 778: 127–134.
Hornyak, M., Cejnar, M., Elam, M., Matousek, M. and Wallin, B. G. Sympathetic muscle nerve activity during sleep in man. Brain, 1991, 114: 1281–1295.
Johnson, L. C. and Karpan, W. E. Autonomic correlates of the spontaneous K-complex. Psychophysiology, 1968, 4: 444–452.
Johnson, L. C. and Lubin, A. Spontaneous electrodermal activity during waking and sleeping. Psychophysiology, 1966, 3: 8–17.
Johnson, L. C. and Lubin, A. The orienting reflex during waking and sleeping. EEG Clin. Neurophysiol., 1967, 22: 11–21.
Jones, B. E. and Webster, H. H. Neurotoxic lesions of the dorsolateral pontomesencephalic tegmentum-cholinergic cell area in the cat. I. Effects upon the cholinergic innervation of the brain. Brain Res., 1988, 451: 13–32.
Jung, R. Correlations of bioelectrical and autonomic phenomena with alteration of consciousness and arousal in man. In: Brain Mechanisms and Consciousness. Selafresnaye, J., Ed. Blackwell, Oxford, 1954: 310.
Karadeniz, D., Ondze, B., Besset, A. and Billiard, M. EEG arousals and awakenings in relation with periodic leg movements during sleep. J. Sleep Res., 2000, 9: 273–277.
Kato, T., Montplaisir, J. Y., Guitard, F., Sessle, B. J., Lund, J. P. and Lavigne, G. J. Evidence that experimentally induced sleep bruxism is a consequence of transient arousal. J. Dent. Res., 2003, 82: 284– 288.
Kaufmann, L. S. and Morrison, A. R. Spontaneous and elicited PGO spikes in rats. Brain Res., 1981, 21: 61–72.
Klemm, W. R. and Naugle, N. W. Oscillatory electrographic activity in the hippocampus: a mathematical model. Neurosci. Biobehav. Rev., 1980, 4: 437–449.
Lavigne, G., Zucconi, M., Castronovo, C., Manzini, C., Marchettini, P. and Smirne, S. Sleep arousal response to experimental thermal stimulation during sleep in human subjects tie of pain and sleep problems. Pain, 2000, 84: 283–290.
Lester, B. K., Burch, N. R. and Dossett, R. C. Nocturnal EEG-GSR profiles: the influence of presleep states. Psychophysiology, 1967, 3: 238–248.
Levine, B., Roehrs, T., Stepanski, E., Zorick, F. and Roth, T. Fragmenting sleep diminishes its recuperative value. Sleep, 1987, 10: 590–599.
Liguori, R., Donadio, V., Foschini, E., DiStasi, V., Plazzi, G., Lugaresi, E. and Montagna, P. Sleep stage-related changes in sympathetic sudomotor and vasomotor skin responses in man. Clin. Neurophysiol., 2000, 111: 434–439.
Lofaso, F., Goldenberg, F., d’Ortho, M. P., Coste, A. and Harf, A. Arterial blood pressure response to transient arousals from NREM sleep in nonapneic snorers with sleep fragmentation. Chest, 1998, 113: 985–991.
Macaluso, G. M., Guerra, P., Di Giovanni, G., Boselli, M., Parrino, L., and Terzano, M. G. Sleep bruxism is a disorder related to periodic arousals during sleep. J. Dent. Res., 1998, 77: 565–573.
McCarley, R. W. and Hobson, J. A. Neuronal excitability modulation over the sleep cycle: a structural and mathematical model. Science, 1975, 189: 58–60.
McCarley, R. W. and Massaquoi, S. G. Neurobiological structure of the revised limit cycle reciprocal interaction model of REM cycle control. Sleep Res., 1992, 1: 132–137.
McCarley, R. W., Winkelman, J. W., and Duffy, F. H. Human cerebral potentials associated with REM sleep rapid eye movements: links to PGO waves and waking potentials. Brain Res., 1983, 274: 359–364.
McDonald, D. G., Shallenberger, H. D., Koresko, R. L. and Kinzy, B. G. Studies of spontaneous electrodermal responses in sleep. Psychophysiology, 1976, 13: 128–134.
MacFarlane, J. G., Shahal, B., Mously, C. and Moldofsky, H. Periodic K-alpha sleep EEG activity and periodic limb movements during sleep: comparisons of clinical features and sleep parameters. Sleep, 1996, 19: 200–204.
McGinty, D. J. and Harper, R. M. Dorsal raphe neurons: depression of firing during sleep in cats. Brain Res., 1976, 101: 569–575.
McKeown, M. J., Humphries, C., Achermann, P., Borbély, A. A. and Sejnowski, T. J. A new method for detecting state changes in the EEG: exploratory application to sleep data. J. Sleep Res., 1998, 7: 48–56.
McNamara, F., Lijowska, A. S. and Thach, B. T. Spontaneous arousal activity in infants during NREM and REM sleep. J. Physiol., 2002, 538: 263–269.
Madsen, P. L., Holm, S., Vorstrup, S., Friberg, L., Lassen, N. A. and Wildschiodtz, G. Human regional cerebral blood flow during rapid eye movement sleep. J. Cereb. Blood Flow Metab., 1991, 11: 502– 507.
Mahowald, M. Hope for the PLMs quagmire? Editorial. Sleep Med., 2002, 3: 463–464.
Maquet, P. and Phillips, C. Functional brain imaging of human sleep. J. Sleep Res., 1998, 7: 42–47.
Maquet, P., Péters, J.M., Aerts
, J., Delfiore, G., Degueldre, C., Luxen, A. and Franck, G. Functional neuroanatomy of human rapid eye movement sleep and dreaming. Nature, 1996, 383: 163–166.
Mariotti, M., Formenti, A., and Mancia, M. Responses of VPL thalamic neurons to peripheral stimulation in wakefulness and sleep. Neurosci. Lett., 1989, 102: 70–75.
Martin, S. E., Engelman, H. M., Kingshott, R. N. and Douglas, N. J. Microarousals in patients with apnoea/hypopnoea syndrome. J. Sleep Res., 1997a, 6: 276–280.
Martin, S. E., Wraith, P. K., Deary, I. J. and Douglas, N. J. The effect of nonvisible sleep fragmentation on daytime function. Am. J. Resp. Crit. Care Med., 1997b, 155: 1596–1601.
Massaquoi, S. G. and McCarley, R. W. Extension of the limit cycle reciprocal interaction model of REM cycle control. An integrated sleep control model. Sleep Res., 1992, 1: 138–143.
Merica, H. and Fortune, R. D. A neuronal transition probability model for the evolution of power in the sigma and delta frequency bands of sleep EEG. Physiol. Behav., 1997, 62: 585–589.
Montplaisir, J. Y. Cholinergic mechanisms involved in cortical activation during arousal. EEG Clin. Neurophysiol., 1975, 38: 263– 272.
Moruzzi, G. The sleep-waking cycle. Ergeb. Physiol., 1972, 64: 1–165.
Moruzzi, G. and Magoun, H. W. Brain stem reticular formation and activation of the EEG. EEG Clin. Neurophysiol., 1949, 1: 455–473.
Mouze-Amady, M., Sockeel, P. and Leconte, P. Modification of REM sleep behavior by REMs contingent auditory stimulation in man. Physiol. Behav., 1986, 37: 543–548.
Nicholas, C. L., Trinder, J. and Colrain, I. M. Increased production of evoked and spontaneous K-complexes following a night of fragmented sleep. Sleep, 2002, 25: 882–887.
Niiyama, Y., Fushimi, M., Sekine, A. and Hishikawa, Y. K. K-complex evoked in NREM sleep is accompanied by a slow negative potential related to cognitive process. EEG Clin. Neurophysiol., 1995, 95: 27–33.
Nofzinger, E. A., Mintun, M. A., Wiseman, M. B., Kupfer, D. J. and Moore, R. Y. Forebrain activation in REM sleep: an FDG PET study. Brain Res., 1997, 770: 192–201.
Novak, P., Lepicovska, V. and Dostalek, C. Periodic amplitude of EEG. Neurosci. Lett., 1992, 136: 213–215.
Ogilvie, R. D., Hunt, H. T., Tyson, P. D., Lucescu, M. L. and Jeakins, D. B. Lucid dreaming and alpha activity: a preliminary report. Percept. Mot. Skills 1982, 55: 795–808.
Oswald, J. Falling asleep open-eyed during intense rhythmic stimulation. BMJ, 1960, 1: 1450–1455.
Paiva, T., Arriaga, F., Rosa, A. and Leitao, J. N. Sleep phasic events in dysthymic patients: a comparative study with normal controls. Physiol. Behav., 1993, 54: 819–824.
Parrino, L. and Terzano, M. G. Polysomnographic effects of hypnotic drug. Psychopharmacology, 1996, 126: 1–16.
Parrino, L., Spaggiari, M. C., Boselli, M., Barusi, R. and Terzano, M. G. Effects of prolonged wakefulness on cyclic alternating pattern (CAP) during sleep recovery at different circadian phases. J. Sleep Res., 1993, 2: 91–95.
Parrino, L., Boselli, M., Buccino, G. P., Spaggiari, M. C., Di Giovanni, G. and Terzano, M. G. The cyclic alternating pattern plays a gate-control on periodic limb movements during non-rapid eye movement sleep. J. Clin. Neurophysiol., 1996, 13: 314–323.
Parrino, L., Boselli, M., Spaggiari, M. C., Smerieri, A. and Terzano, M. G. Multi-drug comparison (lorazepam, triazolam, zolpidem, zopiclone) in situational insomnia: polysomnographic analysis by means of the cyclic alternating pattern (CAP). Clin. Neuropharmacol., 1997, 20: 253–263.
Parrino, L., Boselli, M., Spaggiari, M. C., Smerieri, A. and Terzano, M. G. Cyclic alternating pattern (CAP) in normal sleep: polysomnographic parameters in different age groups. EEG Clin. Neurophysiol., 1998, 107: 439–450.
Parrino, L., Smerieri, A., Spaggiari, M. C. and Terzano, M. G. Cyclic alternating pattern (CAP) and epilepsy during sleep: how a physiological rhythm modulates a pathological event. Clin. Neurophysiol., 2000a, 111: S39–46.
Parrino, L., Smerieri, A., Boselli, M., Spaggiari, M. C. and Terzano, M. G. Sleep reactivity during acute nasal CPAP in obstructive sleep apnea syndrome. Neurology, 2000b, 54: 1633–1640.
Parrino, L., Smerieri, A., Rossi, M. and Terzano, M. G. Relationship of slow and rapid EEG components of CAP to ASDA arousals in normal sleep. Sleep, 2001, 24: 881–885.
Parrino, L., Zucconi, M. and Terzano, M. G. Fragmentation du sommeil chez le patient éprouvant de la douleur. Doul et Analg., 2003, 2: 71–78.
Pavlov, I. P. Lectures on Conditioned Reflexes. International Publishers, New York, 1928.
Peigneux, P., Laureys, S., Fuchs, S., Delbeuck, X., Degueldre, C., Aerts, J., Delfiore, G., Luxen, A. and Maquet, P. Generation of rapid eye movements during paradoxical sleep in humans. Neuroimage, 2001, 14: 701–708.
Penttonen, M., Nurminen, N., Mietinnen, R., Sirviö, J., Henze, D. A., Csicsvári, J. and Buzsáki, G. Ultra-slow oscillation (0.025 Hz) triggers hippocampal after discharges in Wistar rats. Neuroscience, 1999, 94: 735–743.
Perrin, F., Gracia-Larrea, L., Mauguiere, F. and Bastuji, H. A differential brain response to the subject’s own name persists during sleep. Clin. Neurophysiol., 1999, 110: 2153–2164.
Perrin, F., Bastuji, H., Mauguiere, F. and Gracia-Larrea, L. Functional dissociation of the early and late portions of human K-complexes. Neuroreport, 2000, 11: 1637–1640.
Peszka, J. and Harsh, J. Effect of sleep deprivation on NREM sleep ERPs and related activity at sleep onset. Int. J. Psychophysiol., 2002, 46: 275–286.
Pitson, D. J. and Stradling, J. R. Autonomic markers of arousal during sleep in subjects undergoing investigation for obstructive sleep apnea, their relationship to EEG arousals, respiratory events and subjective sleepiness. J. Sleep Res., 1998, 7: 53–59.
Poyares, D., Guilleminault, C., Rosa, A., Ohayon, M. and Koester, U. Arousal EEG spectral power and pulse transit time in UARS and mild OSAS subjects. Clin. Neurophysiol., 2002, 113: 1598–1606.
Quattrocchi, J. J., Shapiro, J., Terrier, R. L. and Hobson, A. Transient cardiorespiratory events during NREM sleep: a feline model for human microarousals. J. Sleep Res., 2000, 9: 185–191
Raynal, D., Montplaisir, J. and Dement, W. C. K-alpha events in hypersomniacs and normals. Sleep Res., 1974, 3: 144.
Rechtschaffen, A. and Kales, A. (Eds). A Manual of Standardized Terminology: Techniques and Scoring Stages of Human Subjects. UCLA Brain Information Service/Brain Research Institute, Los Angeles, 1968.
Rees, K., Spence, D. P., Earis, J. E. and Calverley, P. M. Arousal responses from apneic events during non-rapid-eye-movement sleep. Am. J. Respir. Crit. Care Med., 1995, 152: 1016–1021.
Reese, N. B., Garcia-Rill, E. and Skinner, R. D. The pedunculopontine nucleus–auditory input, arousal and pathophysiology. Prog. Neurobiol., 1995, 47: 105–133.
Riva, L., Bianchi, A. M., Castronovo, V., Oldani, A., Zucconi, M. and Ferini-Strambi, L. Heart rate variability in relation to periodic limb movement (PLM) disorder and cyclic alternating pattern (CAP). Sleep, 2002, 25(abstract supplement): A63.
Roth, M., Shaw, J. and Green, J. The form, voltage distribution and physiological significance of the K-complex. EEG Clin. Neurophysiol., 1956, 8: 385–402.
Ruskin, D. N., Bergstrom, D. A., Kaneoke, Y., Patel, B. N., Twery, M. J. and Walters, J. R. Multisecond oscillations in firing rate in the basal ganglia: robust modulation by dopamine receptor activation and anesthesia. J. Neurophysiol., 1999, 81: 2046–2055.
Sallinen, M., Karrtienen, J. and Lytinen, H. Is the appearance of mismatch during stage 2 sleep related to the elicitation of K-complex? EEG Clin. Neurophysiol., 1994, 91: 140–148.
Sanford, L. D., Morrison, A. R., Ball, W. A., Ross, R. J. and Mann, G. L. The amplitude of elicited PGO waves: a
correlate of orienting. EEG Clin. Neurophysiol., 1993, 86: 438–445.
Saper, C. B., Chou, T. C. and Scammell, T. E. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci., 2001, 24: 726–731.
Sassin, J. F. and Johnson, L. C. Body motility during sleep and its relation to the K-complex. Exp. Neurol., 1968, 22: 133–144.
Schenck, C. H., Pareja, J. A., Patterson, A. L. and Mahowald, M. W. Analysis of polysomnographic events surrounding 252 slow-wave sleep arousals in thirty-eight adults with injurious sleepwalking and sleep terrors. J. Clin. Neurophysiol., 1998, 15: 159–166.
Schieber, J. P., Muzet, A. and Ferierre, P. J. R. Les phases d’activation transitoire spontanées su cours du sommeil normal chez l’homme. Arch. Sci. Physiol. 1971, 25: 443–465.
Sforza, E. and Lugaresi, E. Daytime sleepiness and nasal continuous positive airway pressure therapy in obstructive sleep apnea syndrome patients: effects of chronic treatment and 1-night therapy withdrawal. Sleep, 1995, 18: 195–201.
Sforza, E., Jouny, C. and Ibanez, V. Cardiac activation during arousal in humans: further evidence for hierarchy in the arousal response. Clin. Neurophysiol., 2000a, 111: 1611–1619. Sforza, E., Jouny, C., Prilipko, O. and Ibanez, V. Arousal occurrence during sleep in healthy subjects: evidence from a continuum in the arousal response. Sleep, 2000b, 23: A156.
Sforza, E., Juony, C. and Ibanez, V. Time-dependent variation and autonomic activity during periodic leg movements in sleep. Implications for arousals mechanisms. Clin. Neurophysiol., 2002, 113: 883–891.
Simon, N. R., Manshanden, I. and Lopes da Silva, F. H. A MEG study of sleep. Brain Res., 2000, 860: 64–76.
Sinha, A. K., Smyths, K., Zarcone, V. P., Barchas, J. D. and Dement, D. C. Human sleep – electroencephalogram: a damped oscillatory phenomenon. J. Theor. Biol. 1972, 35: 387–393.
Stepanski, E., Lamphere, J., Roehrs, T., Zorick, F. and Roth, T. Experimental sleep fragmentation and sleepiness in normal subjects. Int. J. Neurosci., 1987, 33: 207–214.
Steriade, M. Brain activation, then (1949) and now: coherent fast rhythms in corticothalamic networks. Arch. Itali. De Biologie., 1995, 134: 5–20.
Steriade, M. Brain electrical activity and sensory processing during waking and sleep states. In: M. Kryger, T. Roth and W. C. Dement (Eds) Principles and Practice of Sleep Medicine. W. B. Saunders, Philadelphia, 2000a: 93–111.
Steriade, M. Corticothalamic resonance, states of vigilance and mentation. Neuroscience, 2000b, 101: 243–276.
Steriade, M. and Amzica, F. Coalescence of sleep rhythms and their chronology in corticothalamic networks. Sleep Res. Online, 1998, 1: 1–10.
Steriade, M. and Llinas, R. The functional states of the thalamus and the associated neuronal interplay. Physiol. Rev., 1988, 68: 649–742.
Steriade, M. and McCarley, R. W. Brainstem Control of Wakefulness and Sleep. Plenum Press, New York, 1990.
Steriade, M., Gloor, P., Llinas, R. R., Lopes da Silva, F. H. and Mesulam, M. M. Basic mechanisms of cerebral rhythmic activities. Report of IFCN Committee on Basic Mechanisms. EEG Clin. Neurophysiol., 1990, 76: 481–508.
Steriade, M., Nunez, A. and Amzica, F. A novel slow (<1 Hz) neocortical oscillation and other sleep rhythms. J. Neurosci., 1993, 13: 3252–3265.
Szűcs, A., Bódizs, R., Barsi, P. and Halász, P. Organic insomnia and frontobasal tumor: a case report. Eur. Neurol., 2000, 46: 54–56.
Szymusiak, R., Shouse, M. N. and McGinty, D. Brainstem stimulation during sleep evokes abnormal rhythmic activity in thalamic neurons in feline penicillin epilepsy. Brain Res., 1996, 713: 253–260.
Szymusiak, R., Steininger, T., Alam, N. and McGinty, D. Preoptic area sleep-regulating mechanisms. Arch. Ital. Biol., 2001, 139: 77– 92.
Takigawa, M., Uchida, T. and Matsumoto, K. Correlation between occurrences of spontaneous K-complex and the two physiological rhythms of cardiac and respiratory cycles. Brain Nerv., 1980, 32: 127–133.
Terzano, M. G. and Parrino, L. Functional relationship between macro- and microstructure. In: M. G. Terzano, P. Halász and A. C.
Declerck (Eds) Phasic Events and Dynamic Organization of Sleep. Raven Press, New York, 1991: 101–119.
Terzano, M. G. and Parrino, L. Evaluation of EEG cyclic alternating pattern during sleep in insomniacs and controls under placebo and acute treatment with zolpidem. Sleep, 1992, 15: 64–70.
Terzano, M. G. and Parrino, L. Clinical applications of cyclic alternating pattern. Physiol. Behav., 1993a, 54: 807–813.
Terzano, M. G. and Parrino, L. T-sleep: an improved method for scoring breathing-disordered sleep. Sleep, 1993b, 16: 285–286.
Terzano, M. G. and Parrino, L. Origin and significance of the cyclic alternating pattern (CAP). Sleep Med. Rev., 2000, 4: 101–123.
Terzano, M. G., Gatti, P. L., Manzoni, G. C., Formentini, E. and Mancia, D. Is the EEG cyclic alternating pattern a true autonomous entity? Analytic study in a case of post-traumatic coma with good prognosis. Eur. Neurol., 1982, 21: 324–334.
Terzano, M. G., Mancia, D., Salati, M. R., Costani, G., Decembrino, A. and Parrino, L. The cyclic alternating pattern as a physiologic component of normal NREM sleep. Sleep, 1985, 8: 137–145.
Terzano, M. G., Parrino, L. and Spaggiari, M. C. The cyclic alternating pattern sequences in the dynamic organization of sleep. EEG Clin. Neurophysiol., 1988, 69: 437–447.
Terzano, M. G., Parrino, L., Anelli, S. and Halász P. Modulation of generalized spike-and-wave discharges during sleep by cyclic alternating pattern. Epilepsia, 1989, 30: 772–781.
Terzano, M. G., Parrino, L., Fioriti, G., Orofiamma, B. and Depoortere, H. Modifications of sleep structure induced by increasing levels of acoustic perturbation in normal subjects. EEG Clin. Neurophysiol., 1990, 76: 29–38.
Terzano, M. G., Parrino, L., Garofalo, P. G., Durisotti, C. and Filati-Roso, C. Activation of partial seizures with motor signs during cyclic alternating pattern in human sleep. Epilepsy Res., 1991a, 10: 166–173.
Terzano, M. G., Halász, P. and Declerck, A. C. (Eds). Phasic Events and Dynamic Organization of Sleep. Raven Press, New York, 1991b.
Terzano, M. G., Parrino, L., Fioriti, G., Spaggiari, M. C., Buccini, G. P. and Depoortere, H. Assessment of noise-induced sleep fragility in two age ranges by means of polysomnographic microstructure. J. Sound Vib., 1993, 162: 339–345.
Terzano, M. G., Parrino, L., Boselli, M., Spaggiari, M. C. and Di Giovanni, G. Polysomnographic analysis of arousal responses in obstructive sleep apnea syndrome by means of the cyclic alternating pattern. J. Clin. Neurophysiol., 1996, 13: 145–155.
Terzano, M. G., Monge-Strauss, M. F., Mikol, F., Spaggiari, M. C. and Parrino, L. Cyclic alternating pattern as a provocative factor in nocturnal paroxysmal dystonia. Epilepsia, 1997a, 38: 1015–1025.
Terzano, M. G., Parrino, L., Boselli, M., Spaggiari, M. C., Di Giovanni, G. and Smerieri, A. Sensitivity of cyclic alternating pattern to prolonged pharmacotherapy: a 5-week study evaluating zolpidem in insomniac patients. Clin. Neuropharmacol., 1997b, 20: 447–454.
Terzano, M. G., Parrino, L., Boselli, M., Smerieri, A. and Spaggiari, M. C. CAP components and EEG synchronization in the first three sleep cycles. Clin. Neurophysiol., 2000, 111: 283–290.
Terzano, M. G., Parrino, L., Smerieri, A., Chervin, R., Chokroverty, S., Guilleminault, C., Hirshkowitz, M., Mahowald, M., Moldofsky, H., Rosa, A., Thomas, R. and Walters, R. Atlas, rules, and recording techniques for the scoring of cyclic alternating pattern (CAP) in human sleep. Sleep Med., 2001, 2: 537–553.
Terzano, M. G., Parrino, L., Rosa, A., Palomba, V. and Smerieri, A. CAP and arousals in the structural development of sleep: an integrative perspective. Sleep Med., 2002, 3: 221–229.
Terzano, M. G.,
Parrino, L., Spaggiari, M. C., Palomba, V., Rossi, M. and Smerieri, A. CAP variables and arousals as sleep EEG markers for primary insomnia. Clin. Neurophysiol., 2003, 114: 1715–1723.
Thomas, R. J. Cyclic alternating pattern and positive airway pressure titration. Sleep Med., 2002, 3: 315–322.
Thorpy, M. J. (Ed.). Disorders of arousal. In: Handbook of Sleep Disorders. Marcel Dekker, New York, 1990: 531–549.
Trinder, J., Padula, M., Berlowitz, D., Kleiman, J., Breen, S., Rochford, P., Worsnop, C., Thompson, B. and Pierce, R. Cardiac and respiratory activity at arousal from sleep under controlled ventilatory conditions. J. Appl. Physiol., 2001, 90: 1455–1463.
Trinder, J., Allen, N., Kleiman, J., Kralevski, V., Kieverlaan, D., Anson, K. and Kim, Y. On the nature of cardiovascular activation at an arousal from sleep. Sleep, 2003, 26: 543–551.
Ujszászi, J. and Halász, P. Long latency evoked potential components in human slow wave sleep. EEG Clin. Neurophysiol., 1988, 69: 516–522.
Waquier, A., Aloe, L. and Decklerck, A. K-complexes. Are they signs of arousal or sleep protective? J. Sleep Res., 1995, 4: 138–143.
Webb, W. B. and Agnew, H. W. Jr. Sleep onset facilitation by tones. Sleep, 1981, 1: 281–286.
Williams, H. L., Hammack, J. T., Daly, R. L., Dement, W. C. and Lubin, A. Response to auditory stimulation, sleep loss and the EEG stages of sleep. EEG Clin. Neurophysiol., 1964, 16: 269–279.
Williams, H. L., Harman,W., Agnew, M. A. Jr and Wilse, B. W. Sleep patterns in the young adult female: an EEG study. EEG Clin. Neurophysiol., 1966, 20: 264–266.
Winkelman, J. W. The evoked heart rate response to periodic leg movements of sleep. Sleep, 1999, 22: 575–580.
Zucconi, M., Oldani, A., Ferini-Strambi, L. and Smirne, A. Arousal fluctuations in non-rapid eye movement parasomnias: the role of arousal instability. J. Clin. Neurophysiol., 1995a, 12: 147–154.
Zucconi, M., Oldani, A., Ferini-Strambi, L., Calori, G., Castronovo, C. and Smirne, S. EEG arousal pattern in habitual snorers with and without obstructive sleep apnea (OSA). J. Sleep Res., 1995b, 4: 107– 112
|








