MODEL iN: Modelling age-related diseases using induced neurons directly reprogrammed from patient’s fibroblasts
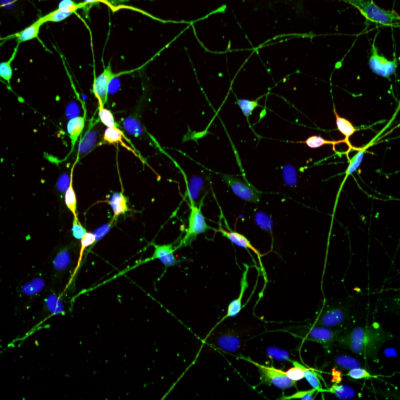
Neurodegenerative disorders are a growing burden in our aging society. With longer life expectancy neurodegenerative disorders will become an even more serious public health issue influencing the active time of the elderly. Accumulating evidence are pointing at impairments in autophagy, a lysosomal degradation pathway, as playing a critical role in ageing and age-related neurodegenerative diseases. However, a clear mechanistic understanding of how alterations in autophagy causes cellular dysfunction and neuronal death, is currently lacking.
The overall aim of this project is to understand how alterations in autophagy contribute to the pathophysiology of chronic incurable neurodegenerative disorders of the central nervous system, such as Alzheimer’s Disease (AD), Huntington’s disease (HD) and Parkinson’s disease (PD). More specifically the aims of this study is to:
i) shed light on impairments of autophagy in neurodegenerative diseases of the CNS,
ii) screen for drugs restoring these defects,
iii) validate the best candidates.
This will be achieved by using models that we have recently developed specifically to study autophagy impairments in neurodegenerative diseases. The model is based on direct neuronal reprogramming of patient derived fibroblasts. This approach will allow to tackle the vulnerability of neurons to different protein build up in a system that is not only of human origin, but also patient specific with carrying the aging signature of the donor.
This information will be instrumental to develop novel treatment strategies for proteinopathies, including Parkinson’s, Huntington’s and Alzheimer’s disease, given their shared alteration in autophagy.
This project is supported by: HCEMM
Neuronal autophagy in human ageing
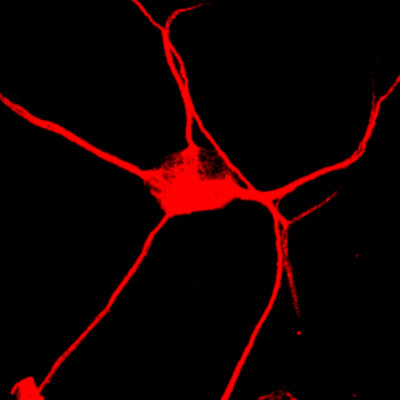
Ageing affects all humans and is the most critical parameter for quality and length of life. Ageing is also by far the most critical risk factor for a majority of neurodegenerative disorders, including Alzheimer’s disease. Accumulating pieces of evidence point at impairments in autophagy, a lysosomal degradation pathway, as playing a critical role in ageing and age-related neurodegenerative diseases. However, a clear mechanistic understanding of how autophagy declines with age and causes cellular dysfunction and neuronal death, is currently lacking due to shortcomings of human neuronal models that recapitulate the ageing signatures of the donor.
The overarching objective of the proposed project is to define how neuronal autophagy declines during physiological healthy ageing in humans.
This project is supported by: OTKA
MiND+: A new drug screening strategy for age-related cognitive disorders using induced neurons directly reprogrammed from patient’s fibroblasts

The world’s population is ageing rapidly and by 2050, the number of people over the age of 60 worldwide is expected to increase from 900 million to 2 billion. Cognitive disorders mainly affect cognitive abilities including learning, memory, perception, and problem solving. The most known form of cognitive disorders is dementia, which affects more than 50 million people up to date worldwide and with the increased life expectancy this number is expected to rise with 10 million new cases every year. Dementia results from a variety of diseases and injuries, such as Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD) or stroke. Dementia is one of the major causes of disability and dependency among older people, which has a tremendous physical, psychological, social, and economic impact, not only on people with dementia, but also on their caretakers, families and the society. New therapeutic strategies to prevent/halt cognitive disorders, must therefore be a focus of current neuroscience research.
In this study, we aim to establish a startup company using an induced neuronal cell culture-based model of cognitive disorders, representing the first model of aged human neurons derived from patients with cognitive disorders, for a drug screening platform. The iN reprogramming methodology will provide a new, robust, quick, easily reproducible drug screening method for novel drug identification/ validation for cognitive disorders.
This project is supported by: TKP-NVA
HUN-REN SZTAKI-SU Rejuvenation Group: Evaluation of human aging and rejuvenation by using artificial intelligence and direct neuronal reprogramming
Aging has a major impact on health, economy and society, but why and how human aging occurs is still poorly understood. Until now, it has not been possible to measure the aging process with precision that can be used for further downstream applications. The recently developed aging clocks, i.e. artificial intelligence models that predict the age of an individual emerged as promising tools for measuring the aging process. The predicted age is an estimate of the biological age of an individual. A value higher or lower than a chronological age can indicate accelerated or slowed aging, which can even highlight a pathological condition years before its clinical manifestation. One of the most important applications of aging clocks is the evaluation and validation of potential rejuvenation treatments. So far, most of the rejuvenation treatments were tested in rodents (rats and mice). Filling this gap, in this proposal with Csaba Kerepesi and his group at the HUN-REN SZTAKI we will be the first to test if young neurons or their environment rejuvenate old human neurons by using induced neurons directly reprogrammed from human dermal fibroblasts. Induced neurons uniquely keep the genetic and the aging signatures of the donor, bypassing any stem cell phase or dedifferentiation during the reprogramming process. In this project we will expose old donor derived induced neurons to a young neuronal milieu and conduct different omics measurements. We will develop specific epigenetic/transcriptomic aging clocks for evaluating biological age and rejuvenation treatments. Our findings will reveal molecular mechanisms behind age-related decline in human neurons and can be the first milestone to discover novel treatments that could maintain or even restore neuronal youth and health, potentially delaying or preventing currently uncurable age-related neurodegenerative disorders.
This project is supported by: TKCS