Patents
-
Benzamide derivatives as anti-inflammatory compounds and uses thereof. WO2021240187. (2021.04.16.)
-
Adam Vannay, Beata Szebeni, Domonkos Pap, Apor Veres-Székely
-
-
Benzamide derivatives as anti-inflammatory compounds and uses thereof. EP20177625.9. (2020.05.29.)
-
Adam Vannay, Beata Szebeni, Domonkos Pap, Apor Veres-Székely
-
-
Composition for organ preservation. WO2018096376. (2017.11.24.)
-
Andrea Fekete, Adam Vannay, Adam Hosszu
-
-
Novel use of Sigma-1 receptor agonist componds. WO2015118365. (2015.08.13.)
-
Andrea Fekete, Adam Vannay
-
Recent scientific articles
All publications at Hungarian Scientific Bibliographi (MTMT)
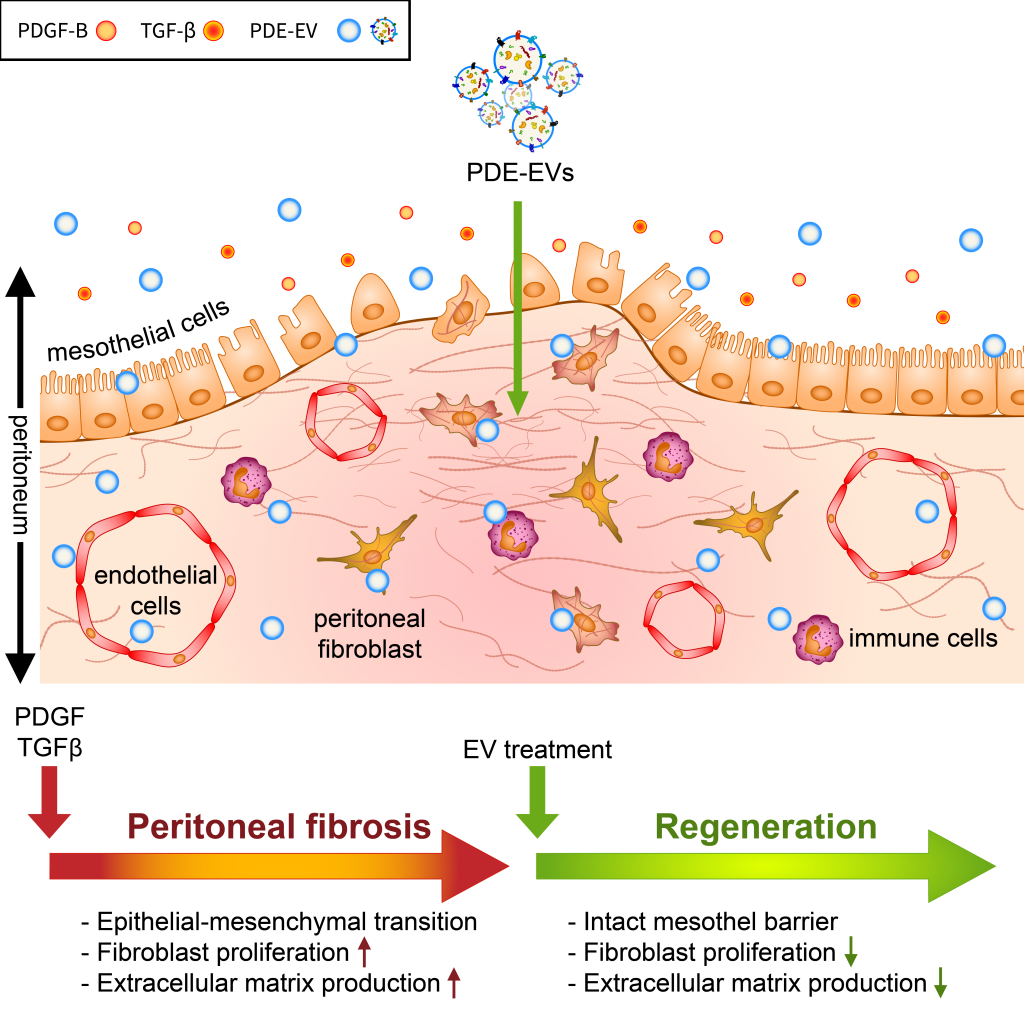
Among patients on peritoneal dialysis (PD), 50–80% will develop peritoneal fibrosis, and 0.5–4.4% will develop life-threatening encapsulating peritoneal sclerosis (EPS). Here, we investigated the role of extracellular vesicles (EVs) on the TGF-β- and PDGF-B-driven processes of peritoneal fibrosis. EVs were isolated from the peritoneal dialysis effluent (PDE) of children receiving continuous ambulatory PD. The impact of PDE-EVs on the epithelial–mesenchymal transition (EMT) and collagen production of the peritoneal mesothelial cells and fibroblasts were investigated in vitro and in vivo in the chlorhexidine digluconate (CG)-induced mice model of peritoneal fibrosis. PDE-EVs showed spherical morphology in the 100 nm size range, and their spectral features, CD63, and annexin positivity were characteristic of EVs. PDE-EVs penetrated into the peritoneal mesothelial cells and fibroblasts and reduced their PDE- or PDGF-B-induced proliferation. Furthermore, PDE-EVs inhibited the PDE- or TGF-β-induced EMT and collagen production of the investigated cell types. PDE-EVs contributed to the mesothelial layer integrity and decreased the submesothelial thickening of CG-treated mice. We demonstrated that PDE-EVs significantly inhibit the PDGF-B- or TGF-β-induced fibrotic processes in vitro and in vivo, suggesting that EVs may contribute to new therapeutic strategies to treat peritoneal fibrosis and other fibroproliferative diseases.
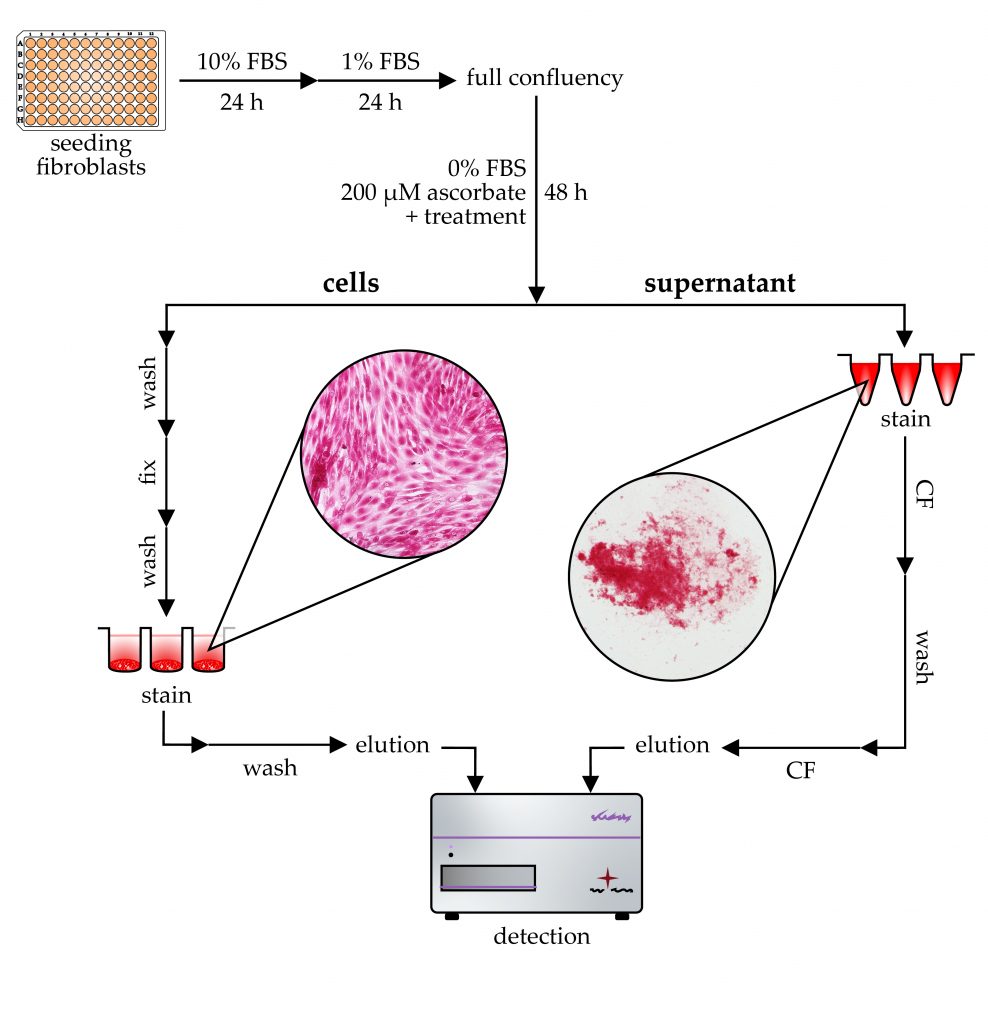
Tissue fibrosis is characterized by chronic fibroblast activation and consequently excessive accumulation of collagen-rich extracellular matrix. In vitro microplate-based assays are essential to investigate the underlying mechanism and the effect of antifibrotic drugs. In this study, in the absence of a gold-standard method, we optimized a simple, cost-effective, Sirius Red-based colorimetric measurement to determine the collagen production of fibroblasts grown on 96-well tissue culture plates. Based on our findings, the use of a serum-free medium is recommended to avoid aspecific signals, while ascorbate supplementation increases the collagen production of fibroblasts. The cell-associated collagens can be quantified by Sirius Red staining in acidic conditions followed by alkaline elution. Immature collagens can be precipitated from the culture medium by acidic Sirius Red solution, and after subsequent centrifugation and washing steps, their amount can be also measured. Increased attention has been paid to optimizing the assay procedure, including incubation time, temperature, and solution concentrations. The resulting assay shows high linearity and sensitivity and could serve as a useful tool in fibrosis-related basic research as well as in preclinical drug screening.
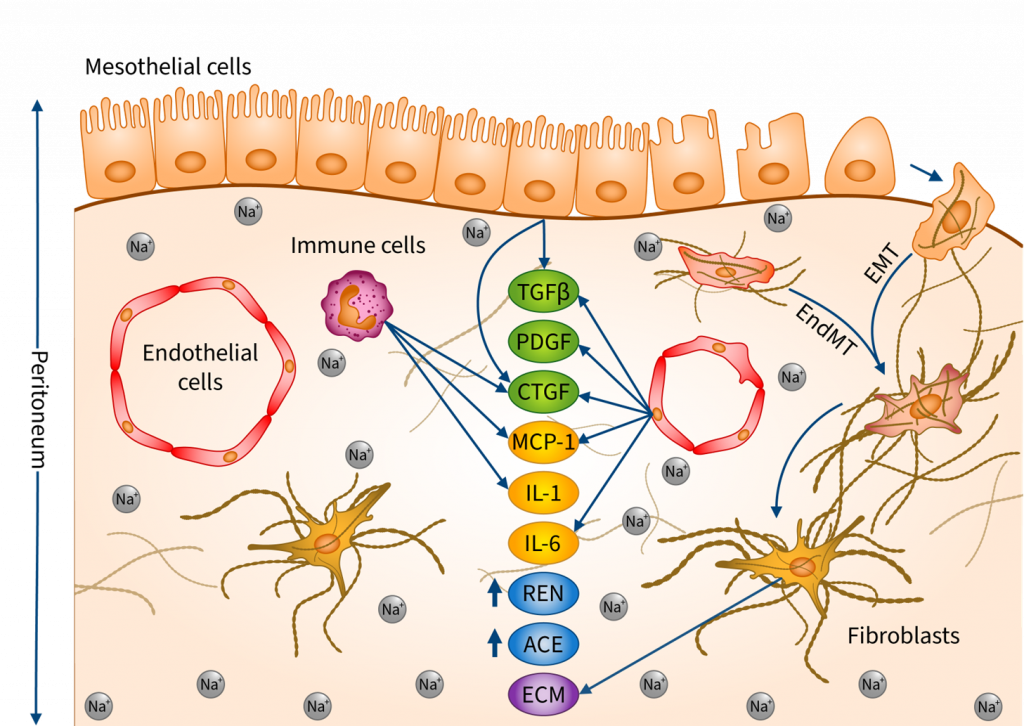
Recent studies draw attention to how excessive salt (NaCl) intake induces fibrotic alterations in the peritoneum through sodium accumulation and osmotic events. The aim of our study was to better understand the underlying mechanisms. The effects of additional NaCl were investigated on human primary mesothelial cells (HPMC), human primary peritoneal fibroblasts (HPF), endothelial cells (HUVEC), immune cells (PBMC), as well as ex vivo on peritoneal tissue samples. Our results showed that a high-salt environment and the consequently increased osmolarity increase the production of inflammatory cytokines, profibrotic growth factors, and components of the renin–angiotensin–aldosterone system, including IL1B, IL6, MCP1, TGFB1, PDGFB, CTGF, Renin and Ace both in vitro and ex vivo. We also demonstrated that high salt induces mesenchymal transition by decreasing the expression of epithelial marker CDH1 and increasing the expression of mesenchymal marker ACTA2 and SNAIL1 in HPMCs, HUVECs and peritoneal samples. Furthermore, high salt increased extracellular matrix production in HPFs. We demonstrated that excess Na+ and the consequently increased osmolarity induce a comprehensive profibrotic response in the peritoneal cells, thereby facilitating the development of peritoneal fibrosis.
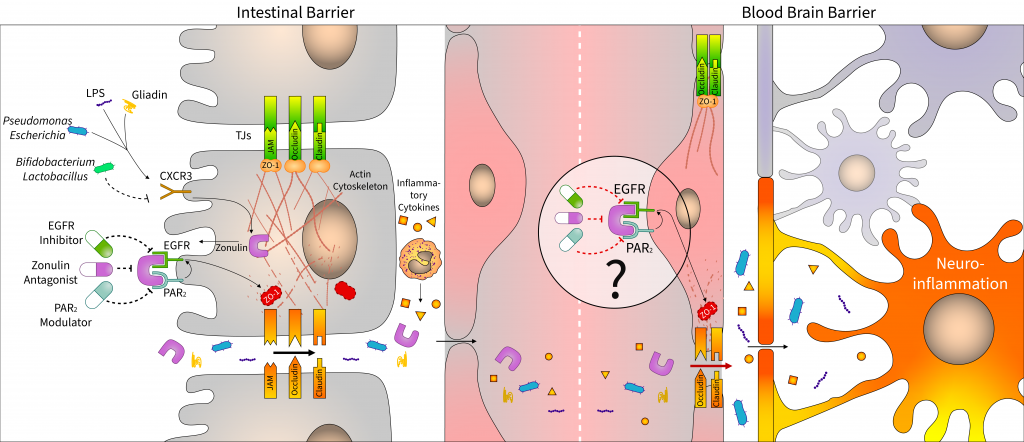
The relationship between dysbiosis and central nervous diseases has been proved in the last 10 years. Microbial alterations cause increased intestinal permeability, and the penetration of bacterial fragment and toxins induces local and systemic inflammatory processes, affecting distant organs, including the brain. Therefore, the integrity of the intestinal epithelial barrier plays a central role in the microbiota–gut–brain axis. In this review, we discuss recent findings on zonulin, an important tight junction regulator of intestinal epithelial cells, which is assumed to play a key role in maintaining of the blood–brain barrier function. In addition to focusing on the effect of microbiome on intestinal zonulin release, we also summarize potential pharmaceutical approaches to modulate zonulin-associated pathways with larazotide acetate and other zonulin receptor agonists or antagonists. The present review also addresses the emerging issues, including the use of misleading nomenclature or the unsolved questions about the exact protein sequence of zonulin.
It is increasingly known that Parkinson’s (PD) and Alzheimer’s (AD) diseases occur more frequently in patients with inflammatory gastrointestinal diseases including inflammatory bowel (IBD) or celiac disease, indicating a pathological link between them. Although epidemiological observations suggest the existence of the gut-brain axis (GBA) involving systemic inflammatory and neural pathways, little is known about the exact molecular mechanisms. Parkinson’s disease 7 (PARK7/DJ-1) is a multifunctional protein whose protective role has been widely demonstrated in neurodegenerative diseases, including PD, AD, or ischemic stroke. Recent studies also revealed the importance of PARK7/DJ-1 in the maintenance of the gut microbiome and also in the regulation of intestinal inflammation. All these findings suggest that PARK7/DJ-1 may be a link and also a potential therapeutic target in gut and brain diseases. In this review, therefore, we discuss our current knowledge about PARK7/DJ-1 in the context of GBA diseases.
Fibroblasts play a central role in diseases associated with excessive deposition of extracellular matrix (ECM), including idiopathic pulmonary fibrosis. Investigation of different properties of fibroblasts, such as migration, proliferation, and collagen-rich ECM production is unavoidable both in basic research and in the development of antifibrotic drugs. In the present study we developed a cost-effective, 96-well plate-based method to examine the migration of fibroblasts, as an alternative approach to the gold standard scratch assay, which has numerous limitations. This article presents a detailed description of our transient agarose spot (TAS) assay, with instructions for its routine application. Advantages of combined use of different functional assays for fibroblast activation in drug development are also discussed by examining the effect of nintedanib—an FDA approved drug against IPF—on lung fibroblasts.
Background: Asymptomatic hyperuricemia is frequently observed in pediatric kidney transplant recipients; symptomatic hyperuricemia, however, is a rare complication. Only few data are available in this patient population. We, therefore, investigated the prevalence of hyperuricemia and its association with kidney transplant function and blood pressure in a multicenter cohort of pediatric kidney transplant recipients. Methods: This is a retrospective, observational multicenter registry study. All pediatric kidney transplant recipients in the CERTAIN database with at least one documented serum uric acid level and a follow-up of 5 years posttransplant were eligible. We identified 151 patients with 395 measurements of serum uric acid. We calculated the prevalence of hyperuricemia, analyzed potential risk factors and clinical consequences such as elevated blood pressure and reduced estimated glomerular filtration rate (eGFR). Statistical analysis was performed using IBM SPSS Statistics 26. Results: One hundred and ten of 395 (27.8%) serum uric acid levels were above 416 µmol/L (7.0 mg/dL), defined as the upper limit of normal. Univariate analysis showed a significant (p = .026) inverse association of serum uric acid with eGFR overtime. There was no significant association of serum uric acid concentrations with body mass index (z-score), blood pressure (z-score), or sex. No episodes of gout were documented. Conclusion: This study shows that hyperuricemia is present in a considerable number of patients sometime after pediatric kidney transplantation and is associated with lower eGFR. Whether hyperuricemia contributes to faster decline of graft function or to the overall cardiovascular risk of these patients remains to be elucidated. Keywords: gout; long-term outcome; pediatric kidney transplantation; uric acid; uric acid-lowering therapy.
Background: Recently, increased interleukin (IL)-24 expression has been demonstrated in the colon biopsies of adult patients with inflammatory bowel disease (IBD). However, the role of IL-24 in the pathomechanism of IBD is still largely unknown. Results: Presence of IL-24 was determined in the samples of children with IBD and in the colon of dextran sodium sulfate (DSS) treated mice. Effect of inflammatory factors on IL24 expression was determined in peripheral blood (PBMCs) and lamina propria mononuclear cells (LPMCs). Also, the impact of IL-24 was investigated on HT-29 epithelial cells and CCD-18Co colon fibroblasts. Expression of tissue remodeling related genes was investigated in the colon of wild type (WT) mice locally treated with IL-24 and in the colon of DSS treated WT and Il20rb knock out (KO) mice. Results: Increased amount of IL-24 was demonstrated in the serum and colon samples of children with IBD and DSS treated mice compared to that of controls. IL-1β, LPS or H2O2 treatment increased the expression of IL24 in PBMCs and LPMCs. IL-24 treatment resulted in increased amount of TGF-β and PDGF-B in HT-29 cells and enhanced the expression of extracellular matrix (ECM)-related genes and the motility of CCD-18Co cells. Similarly, local IL-24 treatment increased the colonic Tgfb1 and Pdgfb expression of WT mice. Moreover, expression of pro-fibrotic Tgfb1 and Pdgfb were lower in the colon of DSS treated Il20rb KO compared to that of WT mice. The disease activity index of colitis was less severe in DSS treated Il20rb KO compared to WT mice. Conclusion: Our study suggest that IL-24 may play a significant role in the mucosal remodeling of patients with IBD by promoting pro-fibrotic processes.
Recent animal studies, as well as quantitative sodium MRI observations on humans demonstrated that remarkable amounts of sodium can be stored in the skin. It is also known that excess sodium in the tissues leads to inflammation in various organs, but its role in dermal pathophysiology has not been elucidated. Therefore, our aim was to study the effect of dietary salt loading on inflammatory process and related extracellular matrix (ECM) remodeling in the skin. To investigate the effect of high salt consumption on inflammation and ECM production in the skin mice were kept on normal (NSD) or high salt (HSD) diet and then dermatitis was induced with imiquimod (IMQ) treatment. The effect of high salt concentration on dermal fibroblasts (DF) and peripheral blood mononuclear cells (PBMC) was also investigated in vitro. The HSD resulted in increased sodium content in the skin of mice. Inflammatory cytokine Il17 expression was elevated in the skin of HSD mice. Expression of anti-inflammatory Il10 and Il13 decreased in the skin of HSD or HSD IMQ mice. The fibroblast marker Acta2 and ECM component Fn and Col1a1 decreased in HSD IMQ mice. Expression of ECM remodeling related Pdgfb and activation phosphorylated (p)-SMAD2/3 was lower in HSD IMQ mice. In PBMCs, production of IL10, IL13 and PDGFB was reduced due to high salt loading. In cultured DFs high salt concentration resulted in decreased cell motility and ECM production, as well. Our results demonstrate that high dietary salt intake is associated with increased dermal pro-inflammatory status. Interestingly, although inflammation induces the synthesis of ECM in most organs, the expression of ECM decreased in the inflamed skin of mice on high salt diet. Our data suggest that salt intake may alter the process of skin remodeling.
Recently the role of Parkinson’s disease 7 (PARK7) was studied in gastrointestinal diseases, however, the complex role of PARK7 in the intestinal inflammation is still not completely clear. Expression and localization of PARK7 were determined in the colon biopsies of children with inflammatory bowel disease (IBD), in the colon of dextran sodium sulphate (DSS) treated mice and in HT-29 colonic epithelial cells treated with interleukin (IL)-17, hydrogen peroxide (H2O2), tumor necrosis factor (TNF)-α, transforming growth factor (TGF)-β or lipopolysaccharide (LPS). Effect of PARK7 on the synthesis of IBD related cytokines was determined using PARK7 gene silenced HT-29 cells and 3,4,5-trimethoxy-N-(4-(8-methylimidazo(1,2-a)pyridine-2-yl)phenyl)benzamide (Comp23)—compound increasing PARK7 activity—treated mice with DSS-colitis. PARK7 expression was higher in the mucosa of children with Crohn’s disease compared to that of controls. While H2O2 and IL-17 treatment increased, LPS, TNF-α or TGF-β treatment decreased the PARK7 synthesis of HT-29 cells. PARK7 gene silencing influenced the synthesis of IL1B, IL6, TNFA and TGFB1 in vitro. Comp23 treatment attenuated the ex vivo permeability of colonic sacs, the clinical symptoms, and mucosal expression of Tgfb1, Il1b, Il6 and Il10 of DSS-treated mice. Our study revealed the role of PARK7 in the regulation of IBD-related inflammation in vitro and in vivo, suggesting its importance as a future therapeutic target.
Background: Recently, the role of IL-19, IL-20 and IL-24 has been reported in renal disorders. However, still little is known about their biological role. Methods: Localization of IL-20RB was determined in human biopsies and in the kidneys of mice that underwent unilateral ureteral obstruction (UUO). Renal Il19, Il20 and Il24 expression was determined in ischemia/reperfusion, lipopolysaccharide, streptozotocin, or UUO induced animal models of kidney diseases. The effects of H2O2, LPS, TGF-β1, PDGF-B and IL-1β on IL19, IL20 and IL24 expression was determined in peripheral blood mononuclear cells (PBMCs). The extents of extracellular matrix (ECM) and α-SMA, Tgfb1, Pdgfb, and Ctgf expression were determined in the kidneys of Il20rb knockout (KO) and wild type (WT) mice following UUO. The effect of IL-24 was also examined on HK-2 tubular epithelial cells and NRK49F renal fibroblasts. Results: IL-20RB was present in the renal biopsies of patients with lupus nephritis, IgA and diabetic nephropathy. Amount of IL-20RB increased in the kidneys of mice underwent UUO. The expression of Il19, Il20 and Il24 increased in the animal models of various kidney diseases. IL-1β, H2O2 and LPS induced the IL19, IL20 and IL24 expression of PBMCs. The extent of ECM, α-SMA, fibronectin, Tgfb1, Pdgfb, and Ctgf expression was lower in the kidney of Il20rb KO compared to WT mice following UUO. IL-24 treatment induced the apoptosis and TGF-β1, PDGF-B, CTGF expression of HK-2 cells. Conclusions: Our data confirmed the significance of IL-19, IL-20 and IL-24 in the pathomechanism of renal diseases. Furthermore, we were the first to demonstrate the pro-fibrotic effect of IL-24.
Coeliac disease (CD) is a chronic, immune-mediated small intestinal enteropathy, accompanied with gluten-triggered oxidative damage of duodenal mucosa. Previously, our research group reported an increased mucosal level of the antioxidant protein Parkinson’s disease 7 (PARK7) in children with CD. In the present study, we investigated the role of increased PARK7 level on the epithelial cell and mucosal integrity of the small intestine. The presence of PARK7 was investigated using immunofluorescent staining on duodenal mucosa of children with CD and on FHs74Int duodenal epithelial cells. To investigate the role of oxidative stress, FHs74Int cells were treated with H2O2 in the absence or presence of Comp23, a PARK7-binding compound. Intracellular accumulation of reactive oxygen species (ROS) was determined by DCFDA-based assay. Cell viability was measured by MTT, LDH, and Annexin V apoptosis assays. Disruption of cytoskeleton and cell adhesion was investigated by immunofluorescence staining and by real-time RT PCR. Effect of PARK7 on mucosal permeability was investigated ex vivo using intestinal sacs derived from control and Comp-23-pretreated mice. Comp23 treatment reduced the H2O2-induced intracellular accumulation of ROS, thus preserving the integrity of the cytoskeleton and also the viability of the FHs74Int cells. Accordingly, Comp23 treatment increased the expression of antioxidants (NRF2, TRX1, GCLC, HMOX1, NQO1), cell-cycle regulators (TP53, CDKN1A, PCNA, BCL2, BAX), and cell adhesion molecules (ZO1, CDH1, VCL, ITGB5) of H2O2-treated cells. Pretreatment with Comp23 considerably decreased the small intestinal permeability. In this study, we demonstrate that PARK7-binding Comp23 reduces the oxidative damage of duodenal epithelial cells, via increased expression of NRF2- and P53-regulated genes. Our results suggest that PARK7 plays a significant role in the maintenance of mucosal integrity in CD.
Background Recently, involvement of IL-19, IL-20 and IL-24 has been reported in inflammatory diseases associated with tissue remodeling. However, their impact on the pathomechanism of coeliac disease (CD) is still completely unknown. Methods Expression of IL19, IL20 and IL24 was measured by real-time RT-PCR, protein amount of IL-24, α smooth muscle actin (α-SMA) and fibronectin (FN) was determined by Western-blot analysis in the duodenal biopsies of therapy naive children with CD and controls. Localization of IL-24 and IL-20RB was investigated by immunofluorescent staining in the duodenal mucosa. Effect of recombinant IL-1β, TNF-α, TGF-β and IL-17 treatment on the expression of IL19, IL20, IL24 and their receptors was investigated by real-time RT-PCR in small intestinal epithelial cells (FHs74Int), in primary duodenal myofibroblasts (pdMFs) and in peripheral blood mononuclear cells (PBMCs). Effect of IL-24 on H2O2 treated FHs74Int cells and on pdMFs was measured by MTT, LDH, Annexin V assays, real-time RT-PCR and by fluorescent microscopy. Results We found increased level of IL-24 (3.3×, p < 0.05), α-SMA (2.4×, p < 0.05) and FN (2.3×, p < 0.05) in the duodenal mucosa and increased expression of IL19 (3.6×, p < 0.05) and IL24 (5.2×, p < 0.05) in the PBMCs of children with CD compared to that of controls. IL-1β was a strong inducer of IL24 expression of FHs74Int cells (9.9×, p < 0.05), pdMFs (552.9×, p < 0.05) or PBMCs (17.2×, p < 0.05), as well. IL-24 treatment reduced the number of apoptotic cells (0.5×, p < 0.05) and decreased the expression of inflammatory factors, including IL1A, IL6 and TNF of H2O2-treated FHs74Int cells. IL-24 decreased the proliferation (0.6×, p < 0.05) of PDGF-B treated pdMFs. Moreover, IL-24 treatment altered the morphology of pdMFs by influencing the size of the angles between stress fibers and the longitudinal axis of the cells (2.0×, p < 0.05) and the expression of cytoskeletal components, including ACTA2, ACTB, VIM, SNAI1 and SNAI2. Conclusion Our results suggest that IL-24 plays a significant role in the maintenance of duodenal mucosal integrity in CD.
The contribution of high sodium intake to hypertension and to the severity of immune-mediated diseases is still being heatedly debated in medical literature and in the lay media. This review aims to demonstrate two conflicting views on the topic, with the first part citing the detrimental effects of excessive salt consumption. Sodium plays a central role in volume and blood pressure homeostasis, and the positive correlation between sodium intake and blood pressure has been extensively researched. Despite the fact that the average of global daily salt consumption exceeds recommendations of international associations, health damage from excessive salt intake is still controversial. Individual differences in salt sensitivity are in great part attributed to this contradiction. Patients suffering from certain diseases as well as other vulnerable groups—either minors or individuals of full age—exhibit more pronounced blood pressure reduction when consuming a low-sodium diet. Furthermore, findings from the last two decades give insight into the concept of extrarenal sodium storage; however, the long-term consequences of this phenomenon are lesser known. Evidence of the relationship between sodium and autoimmune diseases are cited in the review, too. Nevertheless, further clinical trials are needed to clarify their interplay. In conclusion, for salt-sensitive risk groups in the population, even stricter limits of sodium consumption should be set than for young, healthy individuals. Therefore, the question raised in the title should be rephrased as follows: “how much salt is harmful” and “for whom is elevated salt intake harmful?”
Sodium (Na+) can accumulate in the skin tissue, sequestered by negatively charged glycosaminoglycans (GAGs). During dietary salt overload, the amount and charge density of dermal GAG molecules – e.g., hyaluronic acid (HA) and chondroitin sulfate (CS) – increases; however, the regulation of the process is unknown. Previously, it has been demonstrated that the level of cyclooxygenase-2 (COX-2) activity and the content of prostaglandin E2 (PGE2) are elevated in the skin due to high-salt consumption. A link between the COX-2/PGE2 system and GAG synthesis was also suggested. We hypothesized that in dermal fibroblasts (DFs) high-sodium concentration activates the COX-2/PGE2 pathway and also that PGE2 increases the production of HA. Our further aim was to demonstrate that the elevation of the GAG content is ceased by COX-2 inhibition in a salt overloaded animal model. For this, we investigated the messenger RNA (mRNA) expression of COX-2 and HA synthase 2 enzymes as well as the PGE2 and HA production of DFs by real-time reverse transcription PCR (qRT-PCR) and ELISA, respectively. The results showed that both high-sodium concentration and PGE2 treatment increases HA content of the media. Sodium excess activates the COX-2/PGE2 pathway in DFs, and COX-2 inhibition decreases the synthesis of HA. In the animal experiment, the HA- and CS disaccharide content in the skin of male Wistar rats was measured using high performance liquid chromatography-mass spectrometry (HPLC-MS). In the skin of rats receiving high-salt diet, the content of both HA- and monosulfated-CS disaccharides increased, whereas COX-2 inhibition blocked this overproduction. In conclusion, high-salt environment could induce GAG production of DFs in a COX-2/PGE2-dependent manner. Moreover, the COX-2 inhibition resulted in a decreased skin GAG content of the salt overloaded rats. These data revealed a new DF-mediated regulation of GAG synthesis in the skin during salt overload.
Background: Prevalence of fibroproliferative diseases, including chronic kidney disease is rapidly increasing and has become a major public health problem worldwide. Fibroproliferative diseases are characterized by increased expression of α smooth muscle actin (α-SMA) that belongs to the family of the six conserved actin isoforms showing high degree homology. The aim of the present study was to develop real-time PCRs that clearly discriminate α-SMA and ß-actin from other actin isoforms. Results: Real-time PCRs using self-designed mouse, human and rat specific α-SMA or ß-actin primer pairs resulted in the specific amplification of the artificial DNA templates corresponding to mouse, human or rat α-SMA or ß-actin, however ß-actin showed cross-reaction with the housekeeping γ-cyto-actin. We have shown that the use of improperly designed literary primer pairs significantly affects the results of PCRs measuring mRNA expression of α-SMA or ß-actin in the kidney of mice underwent UUO. Conclusion: We developed a set of carefully designed primer pairs and PCR conditions to selectively determine the expression of mouse, human or rat α-SMA and ß-actin isoforms. We demonstrated the importance of primer specificity in experiments where the results are normalized to the expression of ß-actin especially when fibrosis and thus increased expression of α-SMA is occur.
Background/Aims: Congenital obstructive nephropathy (CON) is the main cause of pediatric chronic kidney diseases leading to renal fibrosis. High morbidity and limited treatment opportunities of CON urge the better understanding of the underlying molecular mechanisms. Methods: To identify the differentially expressed genes, microarray analysis was performed on the kidney samples of neonatal rats underwent unilateral ureteral obstruction (UUO). Microarray results were then validated by real-time RT-PCR and bioinformatics analysis was carried out to identify the relevant genes, functional groups and pathways involved in the pathomechanism of CON. Renal expression of matrix metalloproteinase (MMP)-12 and interleukin (IL)-24 were evaluated by real-time RT-PCR, flow cytometry and immunohistochemical analysis. Effect of the main profibrotic factors on the expression of MMP-12 and IL-24 was investigated on HK-2 and HEK-293 cell lines. Finally, the effect of IL-24 treatment on the expression of pro-inflammatory cytokines and MMPs were tested in vitro. Results: Microarray analysis revealed 880 transcripts showing >2.0-fold change following UUO, enriched mainly in immune response related processes. The most up-regulated genes were MMPs and members of IL-20 cytokine subfamily, including MMP-3, MMP-7, MMP-12, IL-19 and IL-24. We found that while TGF-β treatment inhibits the expression of MMP-12 and IL-24, H2O2 or PDGF-B treatment induce the epithelial expression of MMP-12. We demonstrated that IL-24 treatment decreases the expression of IL-6 and MMP-3 in the renal epithelial cells. Conclusions: This study provides an extensive view of UUO induced changes in the gene expression profile of the developing kidney and describes novel molecules, which may play significant role in the pathomechanism of CON.