Abstract
Culturing embryos together in a microdrop of media may improve embryo quality, based on the results of animal studies, however individual identification of the embryos in such a system is not possible. The microwell group culture dish contains 9 or 16 microwells with a minimal well-to-well distance and a specific well morphology that facilitates paracrine and autocrine effects. The microwell group culture dish enables individual identification of the embryos while providing the environment that comes with similar benefits as group culture. Our aim was to investigate whether embryo culture in the microwell group culture dish (Primo Vision Dish, Vitrolife) improves IVF outcomes compared to individual culture in human IVF treatment. Five hundred thirty-two IVF-ET cycles were enrolled in this prospective randomized study in a university hospital. IVF cycles were randomized into microwell group culture and individual culture groups. Primary outcome measure was clinical pregnancy rate and secondary outcome measures were embryo quality, fertilization, implantation, delivery and embryo utilization rates. Fertilization rate in ICSI cycles was significantly higher in the microwell group culture group (70.6% vs. 64.9%, P = 0.001). Clinical pregnancy rate was 50.8% in the group culture and 40.6% in the individual culture (P = 0.022). Live birth rate was 41.5% in microwell and 32.9% in individual culture (P = 0.0496). Embryo utilization rate was higher in microwell group culture than in individual culture (80.6% vs. 75.0%; P < 0.001). Microwell group culture has a beneficial effect on IVF outcome and it also allows following up individual embryo development.
ClinicalTrials.gov: NCT01774006.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Embryos are cultured either in groups or individually during in vitro fertilization (IVF) treatment. In group culture, more embryos are cultured together in the same medium. Single or individual culture means that each embryo is cultured alone in a separate droplet of medium. A higher rate of murine (Lane and Gardner 1992; O’Neill 1997; Kelley and Gardner 2019), bovine (Ieda et al. 2018) and human (Ebner et al. 2010) embryos developed to blastocyst in group culture. The higher cell number was also observed following group culture in murine (Lane and Gardner 1992; O’Neill 1997) and porcine (Stokes et al. 2005) blastocyst. Higher pregnancy rate was achieved following the embryo transfer of murine (Lane and Gardner 1992) or human (Ebner et al. 2010) embryos when they are cultured in groups. Moreover a higher rate of embryo utilization (Rebollar-Lazaro and Matson 2010) have been shown when embryos are cultured in groups rather than individually. On the other hand, some studies showed no clinical benefit of group culture compared to single culture (Spyropoulou et al. 1999). However, none of the studies showed any negative effect of group culture.
The main reason behind single embryo culture in human IVF is to provide a possibility for the individual embryo monitoring. The advantages of group culture may be the effect of the secretion of different substances on the neighboring embryos and on the embryo itself. Paracrine (the effects of one embryo on the other) and autocrine (the effects of the embryo on itself) effects may account for the improved laboratory outcome in group culture (Matsuura 2013).
The microwell culture allows individual identification in group culture and it has been used first for culturing zona-free embryos (Vajta et al. 2000, 2010). Later, the method became commonly used to culture mammalian embryos as well (Vajta et al. 2010). To harvest the alleged benefit of the paracrine effects of group culture, embryos/microwells need to be within a certain distance from each other (Stokes et al 2005; Gopichandran and Leese 2006). Well morphology is also an important feature and an optimally designed, narrow microwell may improve embryo quality (Matsuura 2013; Kelley and Gardner 2017) and blastocyst rates (Vajta et al. 2000, 2008; Sugimura et al. 2010) compared to single culture.
The aim of the present study was to compare the effect of culture conditions on human embryos in individual culture in a conventional culture dish with that of group culture in a microwell group culture dish. For this purpose, we compared the IVF outcome following embryo transfer between embryos cultured under the two conditions.
Materials and methods
Overall study design
This was a prospective, randomized, controlled trial with two arms, performed at the Division of Assisted Reproduction, Department of Obstetrics and Gynecology, Semmelweis University, Budapest, Hungary.
The study compared two routinely used embryo culture practices in a university clinic setting (SE-NOI1-ARO-MF05/1-6). The trial was registered in the ClinicalTrials.gov database (NCT01774006).
We prospectively randomized patients to two groups: group culture in microwells (Primo Vision Dish 3 × 3 wells; Vitrolife, Sweden) or individual culture [60 mm Falcon 3004 (BD, Erembodegem, Belgium)].
The primary end point was clinical pregnancy rate. The secondary endpoints were fertilization rate, embryo quality, implantation rate, live birth rate and embryo utilization rate.
Recruitment and randomization
Infertile couples undergo IVF-ET treatment were recruited. Altogether, 532 patients with ≥ 1 oocytes collected were enrolled in the study (264 cycles in the microwell group culture and 268 in the individual culture group).
Randomization was performed by the embryologist after oocyte collection using a computer generated randomization script. Patients and gynecologists performing the IVF treatment were blinded.
Ovarian stimulation
The gonadotropin-releasing hormone-agonist (GnRH) “long protocol” or multiple dose flexible GnRH-antagonist regimens were used for ovarian stimulation. Transvaginal ultrasound-guided aspiration of follicles was performed at 36 h after hCG administration.
IVF procedure and embryo culture
Laboratory procedures were performed by the same two experienced embryologist using standard protocol (Fancsovits et al. 2015). Oocyte and embryo culture was performed in a culture media product line called “G-series” produced by Vitrolife (Göteborg, Sweden).
Fertilization was performed using conventional IVF or intracytoplasmic sperm injection (ICSI) according to the SOP of the clinic (based on semen quality, patient history and cycle characteristics).
In case of conventional IVF oocytes were placed into the culture dishes after 16–18 h of co-incubation with 3 × 105 progressive motile sperm/ml of culture media.
When ICSI treatment was performed, oocytes were injected following enzymatic removal of cumulus cells and were placed into the relevant culture dish immediately after sperm injection.
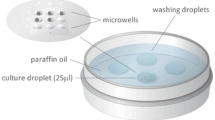
Up to 12 embryos were cultured individually in the conventional culture dish and ≤ 9 embryos were cultured together in the microwell group culture dishes in 25 µl medium droplets (Fig. 1).
Embryo development and morphology were assessed 2 and 3 days after fertilization according to standard protocol (Alpha and ESHRE SIG Embryology 2011). Number of blastomeres, and amount of fragmentation was recorded on day 2 and day 3 of embryo culture. Multinucleated embryos (containing blastomeres with more than one nucleus) were also recorded. The morphology score from 1 to 4 was given to the embryos regarding their quality.
Embryo transfer was performed on day 2 or on day 3 according to patient history and the number of embryos available. Embryo vitrification was performed on day 3 if further normally developed embryos were available.
Outcome measures
Primary endpoint was clinical pregnancy rate, secondary endpoints were fertilization rate, embryo quality, embryo utilization rate, implantation rate and live birth rate. The time needed for the assessment of embryo morphology was also measured.
IVF fertilization rate was calculated as the number of normally fertilized zygotes divided by the total number of oocytes. Fertilization rate following ICSI was determined according to the number of injected oocytes.
Embryo utilization rate was calculated as the number of embryos transferred and cryopreserved, divided by the number of normally fertilized oocytes.
Serum β-hCG levels was measured 11–13 days after ET. Clinical pregnancy was confirmed by the presence of a gestational sac with fetal heart activity 4 weeks after ET. Implantation rate was calculated as the number of gestational sacs with fetal heart activity divided by the number of embryos transferred.
Pregnancies were followed until delivery. The length of pregnancy, the number and birthweight of live newborns and male/female ratio were also recorded. Abortion rates were calculated as the number of clinical pregnancies, while live birth rates were expressed as the number of ETs.
Statistical analysis
Using a two-sided test and Pocock boundaries for estimation, a sample size of 240 patients in each group was calculated (for a total sample size of 480) to achieve a power of 80% at a significance level of 5%. Assuming a 10% dropout rate, a total sample size of 530 (265 in each arm) was needed for the randomized controlled clinical trial.
Student’s t test was used to compare mean values, and the Chi2 test was used to compare proportional values. The time needed for morphology assessment was compared using a paired t-test. Statistical significance was set at P < 0.05.
Results
A total of 532 IVF cycles were involved in the study. The number of cycles was 264 in the microwell culture group and 268 in the individual culture group. The cancellation rate (no embryo transfer due to the absence of fertilization or the elevated risk of ovarian hyperstimulation syndrome) did not differ significantly between the two groups (6.8% vs. 7.1%; χ2(1) = 0.02, P = 0.902). Patient characteristics were similar in the two groups (Table 1).
Semen quality (data not shown), IVF/ICSI ratio and number of Metaphase II oocytes in ICSI cycles were also similar between the two groups (Table 2). For the conventional IVF, the zygotes were placed into culture dishes following 18 h of co-incubation with sperm. Thus, fertilization occurred in the IVF dishes under the same conditions in both groups. On the other hand, for the ICSI cycles, the microinjected oocytes were placed directly into the culture dishes immediately after the sperm injection. Fertilization rates were comparable between the groups when conventional IVF was performed; however, ICSI fertilization rate was significantly higher in the group culture than in the individual culture group (Table 2).
Up to 9 embryos were cultured in the microwell group culture dishes, and the average number of embryos cultured together was 3.4, which resulted in a 7.4 µl/embryo density.
Embryo morphology and the number of good quality embryos were similar between groups; however, the number of blastomeres was significantly higher in the group culture than in the individual culture (Table 3). The number of embryos containing multinucleated blastomeres was slightly lower in group culture than in individual culture dishes.
Clinical pregnancy rate was significantly higher in the microwell culture group than in the individual culture group (50.8% vs 40.6%; χ2(1) = 0.02, P = 0.022); however, implantation rate did not differ significantly between the groups (30.6% vs. 27.2%; χ2(1) = 1.54, P = 0.214; Table 4).
Abortion rate was similar in the two groups (18.4% vs. 18.8%; χ2(1) = 0.01, P = 0.937). Live birth rate was 41.5% in the group culture and 32.9% in the individual culture groups. The difference was slightly significant (χ2(1) = 3.86, P = 0.0496). Length of pregnancy, birth weight of newborns and the male/female ratio did not differ significantly between the groups (Table 4).
Embryos suitable for cryopreservation were available in 44.3% of cycles in the microwell group culture and in 32.9% of cycles in the individual culture group (χ2(1) = 6.76, P = 0.009). Thus, embryo cryopreservation rate and embryo utilization rate were significantly higher in the group culture than in the individual culture (Table 5).
Embryo morphological evaluation needed less time when using the microwell group culture dish compared to a conventional Petri dish (19.2 ± 8.2 s/embryo vs. 23.8 ± 8.2 s/embryo; t(141) = 8.60, P < 0.0001). The average difference between the two groups was 31 s per IVF cycle.
Discussion
Culturing embryos individually in microdrop in a conventional, widely used Petri dish was compared to the group culture of embryos in a microwell group culture dish. The main outcome measure was clinical pregnancy, and further clinically important endpoints were included. Overall, we showed that microwell group culture dish resulted in multiple benefits that were resulted in increased embryo utilization rates as well as increased clinical pregnancy and live birth rates.
The first report on the beneficial effects of group culture in human IVF treatment was published by Moessner and Dodson (Moessner and Dodson 1995). Embryos in group culture may facilitate their own development by producing embryotropins that act as communication messengers. Secretion can be an active procedure, a passive outflow or act via messengers. Inter-embryo communication is regulated by a wide range of biochemical messengers, including growth factors, proteins, lipids, neurotransmitters, saccharides, and microRNAs, all of which can be exchanged among embryos when cultured together in group culture. Different types of messenger molecules influence embryo development by triggering different pathways. Embryotropins, such as insulin-like growth factor-I (IGF-I) or platelet activating factor (PAF), or other molecules, including lipophilic autocrine factors such as prostaglandins or fatty acids, activate different pathways that are important for embryo survival or growth or have anti-apoptotic functions (reviewed by O’Neill 2008; Wydooghe et al. 2015).
According to earlier studies, embryo-to-embryo distance is an important factor affecting the alleged beneficial effects of embryo cross-talk in group culture. It has been shown that distance between embryos cultured together should be < 250 µm (Stokes et al. 2005; Gopichandran and Leese 2006), and embryos outside this distance may not experience any potential effects of group culture. This finding suggests an effective zone of embryotrophic ligands that accumulate around the embryos and may act in autocrine and paracrine manners in group culture (Reed et al. 2011).
In an artificial environment, inter- and intra- embryo communication can be facilitated by modifying culture conditions, eg. introducing microwells with a morphology purposely designed to facilitate the autocrine effect. The volume of the 9-well petridish is 0.11 µl which may result the accumulation of autocrine factors around the embryo (Kelley and Gardner 2017). A study specifically targeting microwell morphology concluded that the concentration of secreted small molecules and macromolecules was two- to threefold higher in a narrow microwell than on a flat surface. The author concluded that the microwell culture system is better than conventional group culture because of the increased final concentration of autocrine factors (Matsuura 2013).
The successful application of narrow and deep microwells that are located in a matrix close to each other has been previously published (Vajta et al. 2000, 2010; Pribenszky 2010a, b) The microwell group culture dish (Fig. 1) allows the individual follow up of embryo development, while narrow and deep (310 µm) wells holding the embryos within 80 µm from each other. This enabled the collection of morphological data from each embryo cultured together in the same dish while obtaining the alleged benefits of group culture due to paracrine and autocrine effects.
An important factor to consider during group culture is embryo density, which is defined as the embryo-to-volume ratio. Generally, 3–15 µl of medium is used per embryo in a group culture of human embryos (Ebner et al. 2010; Rebollar-Lazaro and Matson 2010; Spyropoulou et al. 1999; Moessner and Dodson 1995; Lehner et al. 2017); however, beneficial effects have been reported using either extremely low (< 0.5 µl/embryo) or high (40 µl/embryo) embryo densities in animal studies (Reed et al. 2011). The embryos in our study were cultured in 25 µl of culture medium both in individual and in group culture, results in densities of between 2.8 µl and 25 µl per embryo. This density can supply the nutritional and buffer needs during early embryo development (Gardner et al. 2002).
The fertilization method used may affect the outcome of IVF treatment, however, the fertilization rates following IVF and ICSI treatment were similar in the present study. Overall fertilization rate during the study period achieved the competence values (IVF: 60%, ICSI: 65%) (Alpha and ESHRE SIG Embryology 2011).
We observed a significantly higher fertilization rate in ICSI cycles, while fertilization rate in conventional IVF treatments were the same. The difference in the two fertilization method regarding culture system is that all biological processes following sperm penetration occurred in the respective culture dishes in case of ICSI treatment. We hypothesize that the important physiological procedures after fertilization is enhanced by the fact that zygotes are in a microwell compared to keeping them in a regular Petri dish.
The higher cell number of embryos in group culture refers to a faster embryo development, which can indicate better embryo viability and higher implantation potential. This finding is consistent with the earlier observation of Moessner and Dodson (1995); however, other publications did not confirm these results (Ebner et al. 2010; Spyropoulou et al. 1999). The positive effect of group culture is not evident in terms of embryo morphology during the first 2–3 days of culture (Ebner et al. 2010; Spyropoulou et al. 1999; Vajta et al. 2008). This was confirmed by our study because embryo morphology was similar between the groups. The presence of multinucleation is associated with a lower implantation potential. In our study the number of multinucleate embryos was also lower in microwell culture group.
Previous reports comparing pregnancy and implantation rates in individual and group culture reported different findings. (Rebollar-Lazaro and Matson 2010; Spyropoulou et al. 1999; Ebner et al. 2010; Vajta et al. 2008). The ten percent higher clinical pregnancy rate found in our study indicates that culture in microwell group culture dishes may result in more viable embryos.
The other major advantage of microwell group culture in our study was the higher number of embryos that were available for cryopreservation. Embryo morphology did not differ between the two groups, but the higher cell number and the lower rate of multinucleation might affect the suitability of the embryos for cryopreservation. The number of transferred embryos was similar between the two groups, but more cycles occurred with cryopreservation; thus, the embryo utilization rate was also significantly higher when a microwell group culture dish was used. This finding confirms previous reports, where authors found more blastocysts for cryopreservation and transfer in case of group culture (Rebollar-Lazaro and Matson 2010; Vajta et al. 2008).
The morphological evaluation of embryos in the conventional culture system is time consuming because the distance between microdrops is larger than the field of view of the microscope. In the microwell group culture dish, all of the embryos can be fitted into the same microscope field of view. This can reduce the time needed for embryo evaluation by 30 s on average per patient. Faster evaluation may result in lower fluctuations of temperature and pH during morphological assessment.
Conclusions for future biology
The present study has shown that the use of a microwell group culture dish has a beneficial effect on embryo development and viability in human IVF treatment compared to the conventional individual microdrop culture. The microwell group culture system resulted in higher clinical pregnancy rate and a tendency of higher live birth rate as well. Furthermore, group culture resulted in higher fertilization rate in ICSI cycles and faster embryo development, a higher embryo utilization rate and also reduced the time needed for embryo evaluation compared to individual embryo culture.
References
Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group Embryology (2011) Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Reprod Biomed Online 22:632–646. https://doi.org/10.1016/j.rbmo.2011.02.001
Ebner T, Shebl O, Moser M, Mayer RB, Arzt W, Tews G (2010) Group culture of human zygotes is superior to individual culture in terms of blastulation, implantation and life birth. Reprod Biomed Online 21:762–768. https://doi.org/10.1016/j.rbmo.2010.06.038
Fancsovits P, Lehner A, Murber A, Kaszas Z, Rigo J, Urbancsek J (2015) Effect of hyaluronan-enriched embryo transfer medium on IVF outcome: a prospective randomized clinical trial. Arch Gynecol Obstet 291:1173–1179. https://doi.org/10.1007/s00404-014-3541-9
Gardner DK, Lane M, Schoolcraft WB (2002) Physiology and culture of the human blastocyst. J Reprod Immunol 55:85–100. https://doi.org/10.1016/S0165-0378(01)00136-X
Gopichandran N, Leese HJ (2006) The effect of paracrine/autocrine interactions on the in vitro culture of bovine preimplantation embryos. Reproduction 131:269–277. https://doi.org/10.1530/rep.1.00677
Ieda S, Akai T, Sakaguchi Y, Shimamura S, Sugawara A, Kaneda M, Matoba S, Kagota M, Kaijima H (2018) A microwell culture system that allows group culture and is compatible with human single media. J Assist Reprod Genet 35:1869–1880. https://doi.org/10.1007/s10815-018-1252-z
Kelley RL, Gardner DK (2017) In vitro culture of individual mouse preimplantation embryos: the role of embryo density, microwells, oxygen, timing and conditioned media. Reprod Biomed Online 34:441–454. https://doi.org/10.1016/j.rbmo.2017.02.001
Kelley RL, Gardner DK (2019) Individual culture and atmospheric oxygen during culture affect mouse preimplantation embryo metabolism and post-implantation development. Reprod Biomed Online 39:3–18. https://doi.org/10.1016/j.rbmo.2019.03.102
Lane M, Gardner DK (1992) Effect of incubation volume and embryo density on the development and viability of mouse embryos in vitro. Hum Reprod 7:558–562. https://doi.org/10.1093/oxfordjournals.humrep.a137690
Lehner A, Kaszas Z, Murber A, Rigo J, Urbancsek J, Fancsovits P (2017) Embryo density may affect embryo quality during in vitro culture in a microwell group culture dish. Arch Gynecol Obstet 296:345–353. https://doi.org/10.1007/s00404-017-4403-z
Matsuura K (2013) Numerical calculations for diffusion effects in the well-of-the-well culture system for mammalian embryos. Reprod Fertil Dev 26:742–751. https://doi.org/10.1071/RD13025
Moessner J, Dodson WC (1995) The quality of human embryo growth is improved when embryos are cultured in groups rather than separately. Fertil Steril 64:1034–1035. https://doi.org/10.1016/S0015-0282(16)57925-4
O’Neill C (1997) Evidence for the requirement of autocrine growth factors for development of mouse preimplantation embryos in vitro. Biol Reprod 56:229–237. https://doi.org/10.1095/biolreprod56.1.229
O’Neill C (2008) The potential roles for embryotrophic ligands in preimplantation embryo development. Hum Reprod Update 14:275–288. https://doi.org/10.1093/humupd/dmn002
Pribenszky C, Losonczi E, Molnár M, Lang Z, Mátyás S, Rajczy K et al (2010a) Prediction of in-vitro developmental competence of early cleavage-stage mouse embryos with compact time-lapse equipment. Reprod Biomed Online 20:371–379. https://doi.org/10.1016/j.rbmo.2009.12.007
Pribenszky C, Mátyás S, Kovács P, Losonczi E, Zádori J, Vajta G (2010b) Pregnancy achieved by transfer of a single blastocyst selected by time-lapse monitoring. Reprod Biomed Online 21:533–536. https://doi.org/10.1016/j.rbmo.2010.04.015
Rebollar-Lazaro I, Matson P (2010) The culture of human cleavage stage embryos alone or in groups: effect upon blastocyst utilization rates and implantation. Reprod Biol 10:227–234. https://doi.org/10.1016/S1642-431X(12)60042-4
Reed ML, Woodward BJ, Swain JE (2011) Single or group culture of mammalian embryos: the verdict of the literature. J Reprod Stem Cell Biotechnol 2:77–87. https://doi.org/10.1177/205891581100200203
Spyropoulou I, Karamalegos C, Bolton VN (1999) A prospective randomized study comparing the outcome of in-vitro fertilization and embryo transfer following culture of human embryos individually or in groups before embryo transfer on day 2. Hum Reprod 14:76–79. https://doi.org/10.1093/humrep/14.1.76
Stokes PJ, Abeydeera LR, Leese HJ (2005) Development of porcine embryos in vivo and in vitro; evidence for embryo ‘cross talk’ in vitro. Dev Biol 284:62–71. https://doi.org/10.1016/j.ydbio.2005.05.001
Sugimura S, Akai T, Somfai T, Hirayama M, Aikawa Y, Ohtake M et al (2010) Time-lapse cinematography-compatible polystyrene-based microwell culture system: a novel tool for tracking the development of individual bovine embryos. Biol Reprod 83:970–978. https://doi.org/10.1095/biolreprod.110.085522
Vajta G, Peura TT, Holm P, Páldi A, Greve T, Trounson AO et al (2000) New method for culture of zona-included or zona-free embryos: the Well of the Well (WOW) system. Mol Reprod Dev 55:256–264. https://doi.org/10.1002/(SICI)1098-2795(200003)55:3%3c256::AID-MRD3%3e3.0.CO;2-7
Vajta G, Kőrösi T, Du Y, Nakata K, Ieda S, Kuwayama M et al (2008) The Well-of-the-Well system: an efficient approach to improve embryo development. Reprod Biomed Online 17:73–81. https://doi.org/10.1016/S1472-6483(10)60296-9
Vajta G, Rienzi L, Bavister BD (2010) Zona-free embryo culture: is it a viable option to improve pregnancy rates? Reprod Biomed Online 21:17–25. https://doi.org/10.1016/j.rbmo.2010.03.014
Wydooghe E, Vandaele L, Heras S, De Sutter P, Deforce D, Peelman L et al (2015) Autocrine embryotropins revisited: how do embryos communicate with each other in vitro when cultured in groups? Biol Rev Camb Philos Soc 92:505–520. https://doi.org/10.1111/brv.12241
Funding
Open access funding provided by Semmelweis University. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
PF: Study design, execution, data analysis, manuscript drafting, critical discussion. CP: Study design, manuscript drafting, critical discussion. AL: Study design, execution, data analysis. AM: Execution, manuscript drafting, critical discussion. ZK: Data analysis, manuscript drafting. AN: Manuscript drafting, critical discussion. JU: Study design, execution, critical discussion.
Corresponding author
Ethics declarations
Conflict of interest
Csaba Pribenszky is also active as senior scientist at Vitrolife. All other author has no conflict of interest in relation to this manuscript.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fancsovits, P., Pribenszky, C., Lehner, A. et al. Prospective-randomized study comparing clinical outcomes of IVF treatments where embryos were cultured individually or in a microwell group culture dish. BIOLOGIA FUTURA 73, 229–236 (2022). https://doi.org/10.1007/s42977-022-00113-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42977-022-00113-8